Purpose
High-dose-rate (HDR) brachytherapy is a common administration method in brachytherapy and delivers high doses to the targets in a short time without exposure to technicians. Therefore, this feature increases the need to pay attention to the safety of patients being treated. For example, incorrect source location and dwell time can seriously harm patients. Therefore, efforts to ensure patient safety during HDR brachytherapy are important.
Many events in brachytherapy have been analyzed worldwide. The report of the International Commission on Radiological Protection (ICRP) 97 described HDR brachytherapy events [1] and recommends a method to minimize potential risks such as mechanical, computational, and human errors. Several studies have also analyzed brachytherapy events based on the US Nuclear Regulatory Commission (US NRC) reporting system. Ostrom et al. investigated 35 misadministrations from 1991 to 1992; however, only a few events were related to HDR brachytherapy [2]. Other studies retrospectively analyzed HDR brachytherapy-related events [3,4] and showed that the commonest event was the wrong applicator measurement and the cause was human errors. In addition, using ICRP and US NRC data reported from 2007 to 2011, Nose et al. summarized 64 HDR events for detection timings [5]. Their results revealed that 84% of the events were detected after treatment was administered. Moreover, many of these events were classified as source location-and dwell time-related events. Conversely, 16% of the events were detected during treatment, of which 4 were machine failures.
According to the report of the US NRC Advisory Committee on Medical Uses of Isotopes, among all brachytherapy administered from 2009 to 2010, the incidence of HDR-related events was approximately 0.02% (8 events per 33000 fractions) [6,7]. Another single-institution survey revealed that the incidence of HDR brachytherapy events occurring between 2010 and 2013 was 0.27%, of which 0.15% affected patient safety [8]. Furthermore, according to reports using the recent incident reporting system, the incident rate, including that of incidents, near misses, and programmatic hazards, for 10 years was 1.6% [9].
In December 2013, a misadministration accident for 100 patients with uterine cancer over 7 years was noted in Japan [5]. The cause of this accident was that the input channel length was continuously incorrect; the treatment was performed 3 cm off the tip of the ovoid applicator tip. In Japan, 9 HDR brachytherapy-related events occurred between March 2008 and March 2017 (reported by a single manufacturer’s user community) [10]. Most of those events were classified as occurring because of the wrong dwell position or dwell time of the source. Six of 9 cases were because of human errors. However, one of the cases was because of a source stuck event caused by system malfunction.
In 2014, we experienced a source stuck event when treating patients with cervical cancer. This was the first event in Japan that occurred during HDR brachytherapy treatment. In general, this type of event occurs less frequently than other near-miss events; however, the effect of such events on patients and staff can be more serious. Fortunately, the timely and appropriate response of the staff minimized the situation. Until now, there have been few detailed reports on source stuck events. This study aimed to share our experience and an emergency process for a source stuck event during brachytherapy with other users.
Material and methods
Source stuck event overview
On April 25, 2014, an iridium-192 (192Ir) source stuck event occurred during three-channel HDR brachytherapy treatment for a patient with cervical cancer at our facility. At 8:30 a.m. on the day of the event, a radiation therapy technologist (RTT) performed the morning quality assurance and machine self-check without any problems. We replaced the source on April 16, 2014, of which activity was 403 GBq. At the day of the treatment, the source activity A was 370 GBq. Brachytherapy was initiated at 15:12:07. Treatment with the first and second channels (ovoid applicator) was performed without problems. However, at 15:16:13, the source stuck event occurred during storage of the radiation source after the second channel treatment. Immediately thereafter, the RTT implemented an emergency stop for the device but failed to store the source. At 15:16:33, the RTT entered the treatment room and began to manually collect the source. At 15:16:49, the source storage was successfully completed, and the treatment program was discontinued. The patient and RTT exposure times were estimated to be 36 seconds and 16 seconds, respectively, based on subsequent verifications with the therapy log.
After the event, the safety control staff who received the report notified the hospital director, safety management department, and radiation protection supervisor and vendor. The event was immediately reported to relevant ministries in Japan. The vendors were asked to verify the event, and all treatments with HDR brachytherapy were discontinued until the cause of the event was clarified.
The effect of this event on the patient was immediately estimated. Based on the treatment machine log, the position where the source stuck occurred in the applicator was identified, and the dose distribution owing to the event was estimated using the treatment planning system (TPS). Considering the estimated dose distribution, the remaining treatments of the patient were performed at another hospital. Subsequent follow-up did not confirm the patient’s early and late adverse events. The RTT also immediately conducted individual dosimeter assessment and blood tests to determine the effective dose from exposure and its effects. The RTT exposure dose was below the detection limit of the dosimeter (< 0.1 mSv). In addition, no abnormality was confirmed in the blood data.
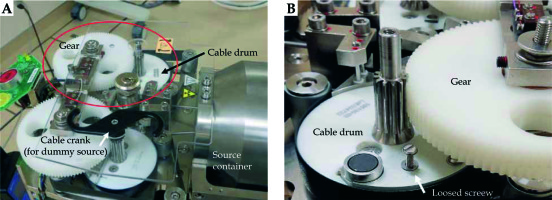
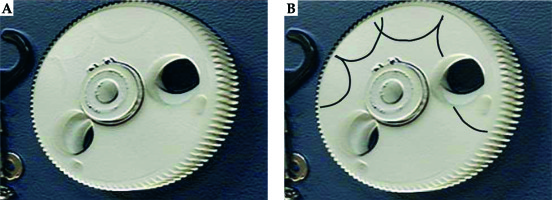
To investigate the accident, 4 types of verification were conducted by the vendor. First, the condition of the source cable was verified. From this analysis, an approximately 26 degree bend was identified at 140 mm from the cable tip. However, this bending was not confirmed to be the cause of the source stuck event. Second, the transfer tube was inspected and slight friction was confirmed in the tube at 1400-1480 mm from the transport start point. However, this friction was not proven to be the cause of the accident. Third, the performance of the emergency circuit of the treatment device was evaluated, and it was confirmed that the circuit functioned properly with reproducibility. Finally, the loose screw found in the cable drum was evaluated. The source cable has a mechanism to wind up by rotating the cable drum and crank (Figure 1A). However, as shown in Figure 2, the gear on the cable drum was discovered to be scratched. Thus, based on the findings of the detailed investigation, the cause of the event was found to be the loose screw that interfered with the gear (Figure 1B). Furthermore, this screw was considered to have been loose 3 months before the event occurred.
Design of normal treatment and emergency response process maps
After the accident was settled, a process map was prepared to respond to normal treatment and emergency events. In these process maps, specific roles were assigned to the staff of the radiation oncology team (radiation oncologist: RO, medical physicist: MP, RTT, nurse: Ns).
A normal brachytherapy process was developed for patients with cervical cancer. The radiation therapy comprised 50.4 Gy/28 fraction external radiation to the entire pelvis and 24.0 Gy/4 fraction HDR brachytherapy to the clinical target volume (CTV) using HDR brachytherapy. During HDR brachytherapy, external radiotherapy with a field that shields the same area depending on the stage is used. We used 2 types of applicators to implement HDR brachytherapy: the tandem and ovoid ones and the cylinder ones. The TPS used was the Oncentra Masterplan Brachy v.3.3 software (Nucletron, an Elekta Company, Elekta AB, Stockholm, Sweden), and the treatment control system (TCS) used was the microSelectron v2 unit (Nucletron, an Elekta Company, Elekta AB, Stockholm, Sweden).
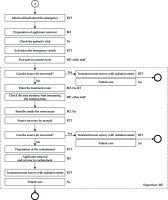
The normal treatment process is shown in Figure 3. This process was conducted by 4 experts: RO, MP, RTT, and Ns. In the treatment planning, CTV, bladder, rectum, intestines, and body surface were delineated by RO. Magnetic resonance images taken in advance were referred to in order to investigate CTV and the surrounding tissue positional relationship. Based on this treatment plan, a dose in which 90% of the CTV is covered (D90) and a dose in which 2 cc of bladder and rectum are covered (D2cc) were identified. The MP calculated the equivalent dose in 2 Gy (EQD2) based on the above parameters. The total dose was estimated from the usual external radiation dose and EQD2 in brachytherapy. In addition, the treatment plan was manually optimized by changing the source dwell time and location. The goals of optimization were as follows: CTV D90 > 87 Gy, rectum D2cc < 75 Gy, and bladder D2cc < 90 Gy. After the treatment plan was complete, the MP performed pre-treatment physical planning checks and independent verifications. Pre-treatment physical planning checks included the selection of appropriate sources, treatment date, catheter length entry, channel assignments, and offset value confirmation. For independent verification, an in-house program that estimated total time using prescription dose, source radioactivity, and prescription volume was used. The difference in total treatment time between the planning and independent calculations was set within 5%. The approved treatment plan was forwarded to the TCS and record verification system. The RO and RTT confirmed the consistency of the treatment time and position between the TCS and TPS. Finally, the RTT confirmed the fixability of the applicator and transfer tube. Once the treatment was initiated, the patient was under the supervision of all staff.
Fig. 3
Process map for cervical cancer treatment. The circle indicates the start of the process. The circle in bold indicates the end of the process. The circle “A” corresponds to an emergency process start. RO – radiation oncologist, MP – medical physicist, RTT – radiation therapy technologist, Ns – nurse

Subsequently, an emergency process map assuming source stuck events was formulated. In emergency situations, staff members were required to immediately move patients away from the source to reduce exposure times, and to facilitate this, staff members were divided into rescue (RO, RTT, and Ns) and recording processes (MP). In this process, the entrance of the treatment room was assumed to be opened to promptly evacuate the patient and staff. Therefore, it was necessary to investigate the effects of radiation outside the treatment room under this special condition and identify a safe evacuation site. Moreover, the three-dimensional dose rate distributions were calculated using the full-scale treatment room and console room models and the new Monte Carlo simulation system.
Dose rate simulation of the treatment room using the FDEIR system
The simulation of the dose rate distribution was performed using the air dose estimation system (Fast Dose Estimation system for Interventional Radiology: FDEIR), which was developed by Takata et al. [11]. The FDEIR system comprises a Monte Carlo method on a graphic processing unit to reduce computation time and the PENELOPE 2006 package for photon transport models. This system enabled the rapid and accurate estimation of the patient’s skin or air dose in the low energy range [11].
The computational geometry was built using 1,852,200 voxels (1 voxel per 4 cm3). Concrete (room walls and floors) and air were assigned as materials, and 2.3 and 0.0012 g cm-3 were applied as physical densities, respectively. We hypothesized that an 192Ir source was present in the air at a height of 1 m from the floor of the central part of the treatment room. Other configuration conditions (e.g., patient and couch, and therapy equipment) were omitted from this simulation to simplify the calculations. We applied 350 keV as the incident energy, which is a well-known average energy of 192Ir. The cutoff energy for photon transport calculations was set to 5 keV, and the number of histories in this simulation was 1 × 1011.
The conversion from the calculation results to the dose rate distribution was performed as follows. Using activity A and the air kerma rate constant Γδ of the source, the air kerma rate 1 m away from the source was calculated as follows: A [GBq] × Γδ [μGy · m2 · MBq-1 · h-1] × 1 [m-2] . Assuming that A = 370 and Γδ = 0.1091 [12], the air kerma rate at a distance of 1 m from the source was calculated to be 4.033 [cGy · h-1] . This value was used for normalization to the calculation result.
To verify the FDEIR simulation, we measured the dose rates of two locations, near the source container (a position 2 m from the source during the treatment) and the internal maze using a calibrated pocket dosimeter (Hitachi PDM-117, Chiba, Japan) and an ionization survey meter (Hitachi ICS-311, Chiba, Japan), respectively. Since two dose rates were estimated to be substantially different at these two locations, we selected the two types of dosimeters which have different range characteristics.
Results
Dose rate distribution in the treatment room
Figure 4 shows the dose rate distributions in the treatment and console rooms calculated by the FDEIR system. The air kerma rates of the internal maze inside the treatment room maze, near the entrance to the treatment room, and near the console room were estimated to be ≅ 10-2 [cGy · h-1], ≅ 10-3 [cGy · h-1], and << 10-3 [cGy · h-1], respectively.
Fig. 4
Dose rate distribution of the 192Ir source in the irradiation and console rooms with the door opened. The black region indicates the concrete region
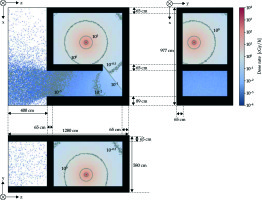
The dose rate near the source container was estimated to be 1.000 ±0.115 [cGy · h-1] under the FDEIR system and 0.935 [cGy · h-1] in actual measurement. Furthermore, the dose rate at the inner maze was estimated to be 4.0 × 10-3 ±1.5 × 10-3 [cGy · h-1] under the FDEIR system and 5.9 × 10-3 [cGy · h-1] in actual measurement.
Details of the emergency response processes from dose rate distribution
The emergency process is shown in Figure 5. This process began by transferring event occurrences from the RTT to other staff members. Three key persons (RO, RTT, and Ns) prepared for patient rescue. Other staff (such as MP) prepared the records and evacuated to the console room. According to the simulation, the console room was confirmed to have almost no effect of the radiation source even when the entrance door to the room was opened. First, the RTT activated the TCS emergency stop mechanism. However, if the mechanism did not function properly, the rescuer entered the treatment room, and thus, the RTT manually attempted to recover the source. Meanwhile, the RO and Ns waited inside the maze of the treatment room where the influence of the radiation source was small. If the radiation source was successfully recovered, the recorder (MP) confirmed that the area monitor’s reading was 0 mSv and the time until source convergence was recorded. Next, the RO removed the applicator from the patient, and the RTT confirmed whether the radiation source remained in the treatment room and patient using a radiation meter. The Ns provided patient care immediately after surveillance. If the source could not be manually retrieved, the RO removed the source from the patient with the applicator and sealed it in a containment vessel. The RTT then surveyed the treatment room and patient to ensure that the source was properly enclosed.
Discussion
Among brachytherapy events already reported, our experience was a rare case. According to reports from the US NRC and ICRP 97 and the Japanese user community, many of the reported events were related to inappropriate channel length, incorrect residence location, incorrect step size, and incorrect prescription volume. Most causes of these events were human errors that were related to treatment planning or commissioning [1,5,10]. Few reports describe equipment malfunction and aftercare in detail. Reports also revealed the incidence of all brachytherapy events to be 0.02-1.6% [6,8,9]. Accordingly, it is assumed that the incidence rate of malfunctioning events is low. We believe that the current detailed report can provide useful information to other brachytherapy users.
In recent years, the application of risk analysis for brachytherapy has spread to mitigate safety problems during treatments. The AAPM TG-100 recommends the use of process maps and failure mode effects analysis (FMEA) and fault tree analysis (FTA) [13]. The benefits of FMEA in radiation therapy have been widely described previously [14,15,16]. FMEA extracts high-risk events by calculating a quantitative score based on 3 parameters (O – occurrence, S – severity, and D – detectability), with each having a range of 1-10. These scores are obtained in the range of 1-1000 risk priority number (RPN) by calculating the product of each parameter value. The plans to reduce high-risk events are formulated against events with high RPN. However, there are no clear criteria for RPN thresholds that result in high-risk events, although there are some reports that define RPN = 80-150 as the threshold [16,17,18].
The event rate that we experienced was low (O = 1 or 2), less detectable (D = 10), and severe (S = 10). As a result, RPN was calculated to be 100 or 200, and consequently, this event was deemed high risk. Traditionally, FMEA needs to identify failure modes from existing clinical processes. However, estimating rare events such as source stuck from the normal treatment process is difficult. Furthermore, high-risk events identified by FTA and FMEA helps list possible causes of occurrence. However, the interference between the structures inside the treatment device that caused this event was in a black box for the user.
The cause of this event was determined to be the interference of loose screws and gears. The loosening of the screw was considered to have occurred 3 months before. However, the quality control conducted on the day of the event and the regular inspections were properly implemented. Unexpected events can occur at any time, even if quality control has been successfully implemented [19]. Naturally, the possibility of occurrence of rare cases such as this event is very low. However, the occurrence of such events cannot be completely avoided. Therefore, it is important to prepare for an emergency response at any time.
We developed the process map to respond to unexpected events after event convergence. In this process, staff members were clearly given roles such as source recovery, patient rescue, and records. This helped minimize the effect on patients and staff by taking prompt and appropriate measures for unexpected events. Furthermore, in formulating this process, we used the dose rate distributions of the treatment and console rooms calculated by the Monte Carlo simulation as a reference. This distribution was used to identify places with a low risk of exposure by performing calculations under special conditions with the door open. We are currently implementing emergency response training based on this process with vendors once a year.
In addition, in Japan, some emergency response manuals were created for HDR brachytherapy users based on our experience. For example, for microSelectron HDR users, a movie was distributed by the vendor that summarized how to handle emergency situations. Moreover, in 2018, a reference book on standard measurement methods of brachytherapy was published by the Japan Society of Medical Physics [20]. An outline of this event was introduced, and a statement was made to recommend the creation of emergency response processes in each institution. In this book, the importance of event awareness and emergency preparation for all HDR brachytherapy users in Japan was underlined.
Some limitations exist in this study. First, the dose rate distribution was simulated in a condition which ignored the structural objects or patient in the treatment room. Since these can be a scatterer or an absorber, actual distribution may be different from the simulated distribution. However, the role of this simulation was to estimate the effect of radiation exposure and to search the safety zone in the maze and the console room. Therefore, we simplified the computational geometry in the treatment room.
Second, the effectiveness of the emergency process and its training have not been proven yet because the frequency of the considered event is extremely low. However, we must always be prepared for the worst-case scenario.
Conclusions
We experienced an 192Ir source stuck event during HDR brachytherapy for the first time in Japan. This event occurred because of the interference of loose screws and gears in the treatment device. Despite the unexpected event, we successfully responded to the accident and minimized the effect on patients and staff. We also created an emergency response process map and are currently conducting staff training based on our experience. The effectiveness of this process and its training remains unconfirmed. Nevertheless, our report will be helpful in managing a patient safety program for other HDR brachytherapy users.