Purpose
The concept of oligo-metastasis [1] based on surgical studies [2,3,4] that was discussed for the first time in the 1990s, differs from the rigid scheme of palliation vs. curation. There is a cohort of oligo-metastasized patients, which is not yet clearly definable that benefits from a consequent local ablation in terms of an improvement in the overall prognosis [5]. The gold standard of local treatment is surgical procedure [6]. However, since a high proportion of hepatic oligo-metastases is not resectable, alternative ablation procedures have been successfully tested [7]. The “toolbox of ablative treatments” is now a part of the current “ESMO (European Society for Medical Oncology) guidelines for the management of patients with metastatic colorectal cancer” [8].
In this study, radio-ablative methods are particularly investigated.
The development of high-performance software for calculation and application of prescribed irradiation dose and device-based hardware, currently allow for very precise implementation of hypo-fractionated and radio-surgical approaches [9,10]. Therefore, in no resectable patient, primary and secondary liver malignancies can often be treated very effectively with radiotherapy [11]. The key for effective and sustainable radio-ablation is to provide adequate clinical target volume doses [12,13], taking into account the dose limits of adjacent organs at risk (OARs). Particularly, in the case of marginal liver tumor, compromises cannot often be avoided at the expense of a potentially reduced chance of local control.
The aim of the present analysis was to investigate the feasibility and safety of a novel approach, in particular, to examine whether an increase in the distance between the target volume and the structure at risk is technically possible without severe complications and to what extent a dosimetric advantage is generated.
Material and methods
Patients
As a rule, all patients who might be eligible for brachytherapy of the liver are considered by a tumor board prior to the initial presentation at our department. A standard operating procedure (SOP) defines the inclusion and exclusion criteria for performing interstitial brachytherapy (iBT) of the liver. All patients sign a written informed consent prior to planning a computed tomography (CT)- or magnet resonance imaging (MRI)-guided interstitial brachytherapy. From April 2009 to June 2016, 2,082 patients with primary or secondary liver tumors were treated with interstitial high-dose-rate (HDR) brachytherapy; 137 cases (6.6%) had subcapsular liver tumors near the stomach, duodenum, or large intestine (OAR).
From this cohort, 31 patients were included in the study and received one or two additional balloon catheter(s) to increase the distance between the hepatic margin/surface and adjacent OAR, as part of single stage CT-guided iBT (recorded dose-volume histogram (DVH) parameters, Table 1 and Figure 1).
Table 1
Recorded dose-volume histogram (DVH) parameters
Fig. 1
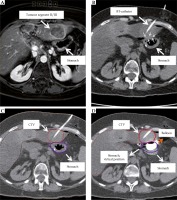
Tomography imaging: A) Transversal MRI-scan: tumor lesion with marginal enhancement of contrast media, no BT, catheter; distinctly adjacent stomach; B) Corresponding transversal CT-scan with stomach position without balloon; one BT, catheter inserted; C) Corresponding transversal CT-scan; CTV and stomach contoured; D) Corresponding transversal CT-scan with additional balloon; CTV, stomach and stomach, virtual position without balloon contoured
The prescribed dose related to D100 depends on the histology of the primary tumor lesion (GIST [gastrointestinal stromal tumor] = 12 Gy, breast cancer, renal cell carcinoma, hepatocellular carcinoma = 15 Gy, other histologies = 20 Gy). The dose was applied as a single fraction targeted on the complete tumor ablation.
Method
Methodology and course of single-dose interstitial HDR brachytherapy was already described in detail elsewhere [12,14].
Briefly, HDR-brachytherapy catheters (Primed, Halberstadt, Germany) and angiographic occlusion balloon catheters (EqualizerTM Occlusion Balloon Catheter, 20 and 27 mm, Boston Scientific, Marlborough, USA) were placed in a similar way using CT fluoroscopy (Aquilion Prime, Canon Medical Systems, Neuss, Germany). Following the puncture of the target lesion (for brachytherapy catheters) or between the liver capsule with the adjacent target lesion and the OAR (for balloon catheters) with an 18-G coaxial needle, a stiff angiography wire (Amplatz Super StiffTM, Boston Scientific, Boston, MA, USA) was introduced for placement of a 6 F (for brachytherapy catheters) or 12 F (for balloon catheters) introducer sheath (Radifocus®, Terumo, Tokyo, Japan), using the Seldinger technique, through which the brachytherapy or balloon catheter was inserted. When in the correct position, the balloon catheter was inflated (with contrast medium) to dissociate the OAR from the target volume (Figure 2). After placement of brachytherapy and balloon catheters, a contrast agent-enhanced (intravenously, iodine-based, 80 ml) spiral CT in breath-holding-technique (slice thickness, 3 mm) of the liver was acquired. The catheter position, the tumor margin, and anatomic risk structures verified by contrast-enhanced images were sent to the treatment planning unit (Oncentra Brachy, Elekta AB, Stockholm, Sweden).
Fig. 2
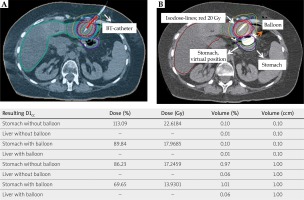
Planning transversal CT scan with isodoses, prescribed dose to D100 CTV 20 Gy: A) CT-scan without balloon, one BT-catheter inserted; B) CT-scan with BT-catheter and one balloon-catheter inserted
The decision to insert a balloon catheter was made after the evaluation of liver specific MRI scans (slice thickness, 3 mm; MRI protocol included: T2-weighted ultra-turbo spin echo sequences with and without fat saturation, diffusion-weighted imaging, a T1-weighted gradient echo sequence, T1-weighted dynamic sequences, and sequences acquired 20 min after IV administration of 0.1 ml/kg Gd-EOB-DTPA [Primovist®, Bayer Vital, Leverkusen, Germany] performed on an 1.5-tesla MRI scanner [Intera 1.5T, Philips Healthcare, Hamburg, Germany], if within the framework of a virtual catheter application, the calculated clinical target volume (CTV) enclosing prescription dose (D100) did not seem to be feasible under consideration of the institutional OAR dose limits concerning D1cc and V5 [13,15,16], and outstanding publications and reviews, inter alia, by Timmermann, Herfarth et al. and Sterzing et al. [17,18,19] (Table 2).
Table 2
Dose constraints regarding organs at risk for single dose
| Organ at risk | Timmermann SBRT constraints [17] | Herfarth, Sterzing, SBRT constraints [18,19] | Institutional constraints due to prospective and retrospective analysis of the XX/YY study-group [13,15,16] | |||
|---|---|---|---|---|---|---|
| DVH-parameter | Limit (Gy) | DVH parameter | Limit (Gy) | DVH parameter | Limit (Gy)/(%) | |
| Stomach | D10cc | < 13.0 | Dmax | 12.0 | D1cc | 14 (15*) |
| Duodenum | D5cc | < 8.8 | Dmax | 12.0 | D1cc | 14 (15*) |
| Colon | D20cc | < 11.0 | Not specified | Not specified | D1cc | 18 |
| Liver | D700cc | 9.1 | D50 | 4.0-7.0 | V5 | /66 |
* The original values based on Streitparth’s work [13] were decreased to 14 Gy from 2012 to further reduce the risk of late toxicity.
The time for insertion of one balloon catheter corresponds approximately to the application time of two BT catheters (mean, 16 min). In case of an implant with one BT catheter tripling the intervention time and in case of more advanced liver lesions with 8 catheters, the duration time of the intervention increases by approximately 25%.
In addition to CTV, liver and adjacent OAR (predominantly stomach) as well as virtual OAR volume without a balloon were contoured; the virtual position of the OAR could be anticipated by assessing the pre-interventional MRI scans and additionally, with the interventional CT scans with BT catheter only (Figure 1).
Dose calculation was performed in strict accordance with institutional OAR limits (Table 2). The relevant parameters for this analysis such as prescription dose, D100-CTV, D1cc-OAR with and without a balloon were recorded. The values for the D1cc-OAR with and D1cc-OAR without balloon were distinguished as two groups and statistically evaluated.
The values for D1cc-OAR with and D1cc-OAR without balloon were assigned to two groups. These two cohorts were compared statistically.
Interstitial HDR brachytherapy was performed using an 192Ir source with an afterloading device from Elekta (MicroSelectron HDR V3, Oncentra Brachy, Elekta AB, Stockholm, Sweden).
Statistics
Statistics were collected with R (version 3.1.3; the R Foundation for Statistical Computing, Vienna, Austria).
Due to small sample size, non-parametric distribution of data was assumed, and data were described by median, interquartile range (IQR, 25th-75th percentiles), and minimum and maximum. Boxplots were used for visualization of data. Correlation of data was analyzed with Spearman’s rho rank correlation coefficient and agreement of methods was described using Bland-Altman analysis [20]. Paired groups (with/without balloon) were compared with Wilcoxon signed rank test, and optimal cut-off was determined using receiver operating characteristics (ROC) curves [21] and Youden index as appropriate. All tests were two-sided, and the significance level was set as 0.05.
Statement
The study was performed according to the guidelines of the Declaration of Helsinki for Biomedical Research from 1964 and its further amendments, and the procedures of “Good Research Practice”. The analysis was designed as a retrospective study with approval of the local ethics committee. Each patient signed a written consent form prior to the planned intervention after an adequate patient-physician talk on the intervention and the frequency, severity, and profile of its complications.
Results
Patients
Thirty-one patients (17 females, 14 males; median age, 65.3 [range, 38-85] years), 22% of those with subcapsular liver tumors, were enrolled in the study. In 25 cases, one in 6 cases, two balloon catheters were inserted.
In 74% of the patients, primary lesions outside the liver were histologically confirmed (colorectal carcinoma, 45%; others, 29%), 26% had primary liver malignancies.
The marginal hepatic lesions were located within the liver segments 2/3 in 29 cases (93.5%), 2 patients had lesions within the right hepatic lobe, near large intestine. Patients’ characteristics are presented in Table 3.
Table 3
Patients’ characteristics
Application time for the whole implant depended on the number of inserted BT catheters and additional balloons. Median application time was 12.5 min (range, 7.5-30 min).
Organs at risk (stomach/duodenum, large intestine) D1cc
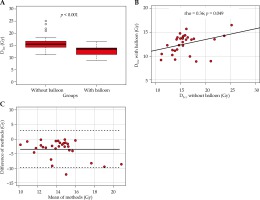
D1cc of the OAR with balloon (mean, 12 Gy; deviation, 8.9 to 16.5 Gy; median, 13.5 Gy; IQR, 11.2 to 14.0 Gy) were significantly (p < 0.001) lower compared to virtual anticipated OAR without a balloon (mean, 16 Gy; deviation, 11.1 to 25.1 Gy; median, 15.5 Gy; IQR, 14.3 to 16.7 Gy; Figure 3A). The corresponding median relative difference was –16.3% (IQR, –23.2 to –8.9%), ranging from –57.3% to –0.7% (Table 4). Figures 3A and 3B shows the correlation of D1cc with and without a balloon, with a Spearman’s correlation coefficient of 0.36 (p = 0.049). Comparing both methods with Bland-Altman, analysis revealed 95% limits of agreement of –9.6 Gy to 2.9 Gy, with a mean of –3.4 Gy (Figure 3C).
Table 4
Statistics: organ at risk (OAR) D1cc with and without a balloon as well as absolute and relative differences
Acute side effects and late morbidity
The additional balloon catheter was tolerated very well by all patients. Serious acute complications (e.g., bleeding) did not occur in any case. During the further course, 4 late complications in 3 patients (1 × abscess, 2 × gastric ulcers, 1 × non-classic radiation-induced liver disease [RILD]) were observed. Complications are described in detail in Table 5.
Table 5
Side effects
| Acute and late side effects according to CTCAE# v. 4.03 [1-5] | Number of cases (n/%) | Patient study number | Treatment/outcome | Interval between iBT and side effect |
|---|---|---|---|---|
| Temporarily increase of bilirubin [°1] | 1/3 | 7 | No treatment/resolved | 24 h |
| Shivering [°1>] | 1/3 | 15 | No treatment/resolved | 1 h |
| Nausea/vomiting [°2] | 2/6 | 29 | Antiemetic drugs/ resolved | 1 h |
| Abscess [°3] | 1/3 | 20 | Drainage and antibiotics/resolved | 8 weeks |
| Non classic RILD## (previous SIRT*) [°3] | 1/3 | 7 | Ursodeoxycholic acid/resolved | 12 weeks (18 weeks after radioembolization) |
| Ulcus ventriculi** [°4] | 1/3 | 20 | Gastrectomy/resolved | 14 weeks |
| Ulcus ventriculi*** [°5] | 1/3 | 11 | Gastrectomy/death | 15 weeks |
Thus, formally the rate of significant late effects was 12.9% (> 2) and 6.45% (> 3), respectively. Of these, only in one case (3.22%, patient no. 20) a severe adverse event (SAE) can be suspected due to repeated radiation exposure of the gastric mucosa. Patient no. 11 suffered from diabetes mellitus and pre-existing chronic gastritis, and received long-term treatment with Avastin® (Bevacizumab, Roche Pharma AG, Grenzach-Wyhlen, Germany) and anticoagulation, whereas patient no. 7 underwent a radio-embolization 18 weeks prior to RILD.
Discussion
The data of this study demonstrate that the interventional application of one or two balloon catheter(s) into the connective tissue layer between the hepatic capsule and adjacent OAR generates a distance between subcapsular tumor lesion of the liver and OAR, resulting in a significant median reduction of dosage exposition of the adjacent OAR of about 16%. This effect enlarges the therapeutic “window” and consecutively, the CTV can be treated with a higher, thus presumably more efficient irradiation dose.
The current ESMO guideline for the treatment of metastatic colorectal cancer (CRC) [8] indicates the growing acceptance of minimally invasive methods for the treatment of oligo-metastases. The so-called “toolbox of minimally invasive methods” is particularly important because a significant proportion of patients with oligo-metastases are not resectable for various reasons [22]. However, in addition to the indisputable role of systemic treatment [23], local control is the key to potentially sustained improvement in the overall prognosis.
Modern irradiation techniques (e.g., stereotactic body radiotherapy [SBRT], iBT) enable precise application of very high single doses. In this regard, in addition to the tumor cell destruction mechanisms based on DNA damage, further effective radiobiological effects can be initiated [24,25]. Though, even the most accurate dose application can be limited by the proximity of sensitive OAR. Chang et al. [26] reported a rate of ≥ 3 toxicity of 10% (mainly gastrointestinal [GI] ulceration) after 25 Gy single fraction SBRT for unresectable pancreatic adenocarcinoma, within adjacent stomach and further GI structures.
The concept of simultaneously integrated protection (SIP) could be a conceivable strategy to avoid high doses to an OAR [27]. Whether this is associated with an increased rate of local recurrences is yet to be seen. This question is currently being examined by a prospective clinical study. Therefore, the possibility of increasing distance of the CTV to surrounding OAR appears promising.
In recent years, various groups [28,29,30] have tested feasibility, safety, and application effect of absorbable polyethylene glycol (PEG) to increase the distance between the prostate and the rectal wall. In fact, by applying PEG, a dosimetrically effective distancing can be achieved.
Thus, higher irradiation doses in patients with prostate cancer can be accomplished without an increased risk of chronic side effects onto the rectal wall. Considering this successful principle of distancing, the analysis presented here verified the feasibility, tolerability, safety, and efficacy of a balloon catheter-based approach.
As a limitation, direct comparison of both approaches, with regard to acute side effects and late toxicities is difficult, since the affected OAR within the pelvis region on one hand and the abdominal cavity on the other have different tolerance doses and, moreover, the total and single doses of the irradiation concepts are not comparable.
In addition, in recent years, numerous studies have been published regarding interstitial brachytherapy of the liver [12,13,30,31,32,33,34,35,36]. The rate of side effects ≥ 3 listed in these studies was approximately 5%.
In contrast, the rate of late toxicities ≥ 3 (12.9%) in this study appears to be higher in comparison to the cited studies. Can one or two additionally applied balloon catheter(s) cause this difference? This is rather unlikely because in the affected patients, the pre-treatment modes (selective internal radiotherapy, surgical procedures, chemotherapy, repeated irradiation) as well as severe co-morbidities (insulin-dependent diabetes mellitus, chronic gastritis etc.) must be taken into consideration. Moreover, the intraoperative situs of the second (gastrectomized) patient (no. 11) also showed a recurrent liver metastasis, which had infiltrated and damaged a large area of the wall of the reconstructed upper GI tract.
Thus, the iBT (plus balloon)-related complication rate summarizing all side effects ≥ 3 (according to CTCAE v. 4.0) would be formally 3% (patient no. 20 with ulcer 4).
A further limitation of the study is the moderate number of cases and the retrospective and monocentric character of the analysis. In addition, the balloon catheters used are not optimal because they cannot distance the adjacent OARs in large space, only in very circumscribed areas. However, as far as known, there is currently no report on increasing the distance between tumor lesion and adjacent OAR by balloon catheter(s).
For optimization, reusable balloon catheters should be designed to be inflated and deflated when in position. In order to avoid selection bias, the results of this analysis should be examined in a prospective, possibly multicenter study.
Conclusions
Insertion of balloon catheters to increase the distance between subcapsular liver malignomas and adjacent OAR is feasible, low-risk (i.e., safe), and minimally invasive to significantly reduce the radiation dose exposure of the affected OAR due to iBT. This distancing of the adjacent OAR allows a higher D100 value of the CTV, therefore allowing for more efficient local control. Consequently, efficacy and sustainability of radio-ablative procedures can be increased.
During a short-term single-fraction iBT, an additional balloon catheter is well tolerated. Whether the insertion of such a catheter would also be possible for a longer period of several days within a fractional SBRT (several days) is currently still not investigated by a systematic study approach.
Thus, the insertion of a balloon catheter in cases with close-fitting OAR, which also overcomes the limitations of percutaneous, non-interventional SBRT, should be further discussed and more extensively proven as an additional option.
Addendum
This work has been conducted without research support.
Results of an interim analysis of this study with 20 patients were presented at the DEGRO-Congress (Hamburg) in 2015, final results at the ESTRO-Congress in Barcelona 2018.
Disclosure
Authors report no conflict of interest.
Dr. Hass reports personal fees from Merck Serono and BMS outside the submitted work.
Dr. Seidensticker reports personal fees from Bayer, grants and personal fees from SIRTEX Medical, personal fees from Cook Medical, personal fees from BTG, outside the submitted work.