Purpose
Low-dose-rate (LDR) brachytherapy is one of the curable treatment options for low-risk prostate cancer, with biochemical-free progression rates of more than 90% up to 10 years post-treatment [1]. In a case of locally recurrent disease after previous radiotherapy (e.g., external beam or brachytherapy), salvage therapy remains very challenging, with theoretical high toxicity risks [2-4]. Majority of such patients are offered palliative hormonal therapy, and only minority, i.e., approx. 2%, are offered treatment with curative intent [2, 5]. Salvage treatment options after previous radiation therapy include salvage cryotherapy, high-intensity focused ultrasound (HIFU) prostatectomy, and LDR and high-dose-rate (HDR) brachytherapy. Salvage cryotherapy and HIFU are associated with low complication rates, but also with poor local control outcomes, demonstrating 40-50% biochemical control rates at 2 to 5 years [6-8]. On the other hand, salvage prostatectomy is associated with slightly better oncologic outcome, with 50-60% biochemical disease-free survival at 5 years, but is associated with substantial toxicity rates, especially urinary incontinence and erectile dysfunction [7, 8]. Moreover, grade 3 urinary incontinence rate after salvage prostatectomy is 41% vs. approx. 6% after salvage brachytherapy [7]. Salvage LDR brachytherapy seems to be effective in disease control, with a 5-year prostate specific antigen (PSA) relapse-free survival rates of 60-71%, but with strongly varying late grade 3 genitourinary (GU)/gastrointestinal (GI) toxicity profiles ranging between 0% and 30% [8-11]. Similar results have been observed with salvage HDR with biochemical control rates between 51% and 77% at 5 years post-treatment [12-15]. Focal salvage therapy instead of re-irradiating the whole gland may result in a reduced toxicity with equal disease control [16].
In this paper, we present a case of a focal salvage HDR treatment after previous iodine-125 (125I) seed implantation, with grade 3 late rectal toxicity for an initial low-risk prostate carcinoma. In this case, we used a rectal balloon spacer implant (RBI) in order to lower the rectal dose, thereby creating a window of opportunity to treat this patient with a curative intent. Without RBI, this patient would probably be offered palliative hormonal treatment.
Case presentation
A 76-year-old male patient was diagnosed in 2013, with a Gleason 3+3 adenocarcinoma of the prostate, with a tumor load of 1 out of 4 biopsies on the left lobe and 1 out of 3 on the right, tumor percentage 30% and 15-20%, respectively. Patient was staged as a cT1cN0M0 and diagnosed after a routine blood measurement (initial PSA, 4 ng/ml). Trans-rectal ultrasound showed a prostate volume of 22 cc. Treatment consisted of LDR brachytherapy with 125I seed implantation, with a prescribed dose of 145 Gray (Gy). Intra-operative dosimetry was as follows: the prostate D90 (dose to the 90% of volume) was 153.3 Gy, 105% of the prescribed dose of 145 Gy. The rectum constraints were relatively low: D0.1cc, D1cc, and D2cc (dose to 0.1 cc, 1 cc, and 2 cc of the rectum) was 156.3 Gy (107.8%), 122.3 Gy (84.4%), and 104.9 Gy (72%), respectively. Two years after seed implantation, the patient presented with rectal hemorrhage, reported as a grade 3 according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 [17]. He was treated with different medical interventions, including budesonide foam, and pentoxifylline and hyperbaric oxygenation therapy in 2016 [18]. By November 2017, the rectal hemorrhage almost completely resolved.
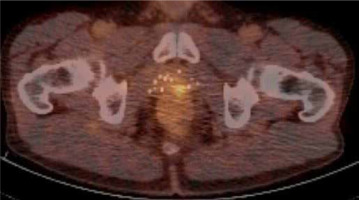
In July 2018, he presented with 3 consecutive PSA rises, according to the ASTRO definition of biochemical recurrence disease, highest value 2.4 ng/ml after nadir PSA at 0.7 ng/ml. Prostate-specific membrane antigen (PSMA) nuclear examination using positron emission tomography (PET) combined with computerized tomography (CT) scan revealed local recurrent disease against the anterior rectal wall, without lymph node involvement or distant metastases (Figure 1). Biopsies showed a Gleason score 4+3 in 2 out of 5 biopsies, with a tumor percentage of 10%.
The patient was discussed at a multi-disciplinary tumor board and because of previously reported high toxicity following primary treatment, external beam re-irradiation was considered as very high-risk. The patient was counselled for salvage HDR brachytherapy with a RBI. The patient underwent a sigmoidoscopy by a gastroenterologist to rule out any deep ulceration, which was considered a risk factor for serious rectal injury following the placement of RBI. Sigmoidoscopy showed residual non-bleeding telangiectasia, without inflammation or ulceration. Prior to salvage treatment in November, 2018, the most recent PSA was 2.7 ng/ml. The multi-disciplinary tumor board and the patient agreed to proceed with aggressive curable treatment.
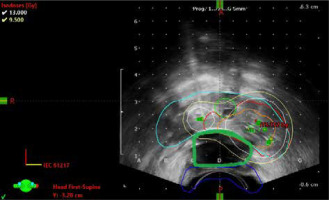
First, a RBI was implanted to maximally decrease the radiation dose to the rectum. The RBI (ProSpace™, BioProtect Inc., Kfar-Saba, Israel) was implanted transperineally between the Denonvilliers’ fascia and the rectum under trans-rectal ultrasonography guidance. The injection technique has been described previously [19]. The RBI was placed just before the start of the first HDR procedure in November, 2018. Gross tumor volume (GTV) was delineated on ultrasound imaging using PSMA-PET CT scan. The margin for clinical target volume (CTV) was 3 mm according to a local protocol. The urethra and the RBI were excluded from CTV (Figure 2). Specifications of the two HDR sessions are presented in Table 1 [20]. Volumes of the prostate and the treated volumes of GTV and CTV were 26.6, 2.8, and 5.9 cc, respectively.
Table 1
Dosimetric specifications of two HDR fractions, one week apart from each other
| Fraction number | D90 GTV | EQD2 (α/β 1.5 [20]) |
|---|---|---|
| One | 12.6 Gy | 50.8 Gy |
| Two | 13.1 Gy | 54.6 Gy |
| Cumulative | 25.7 Gy | 105.4 Gy |
| Rectal dose 2 cc | EQD2 (α/β 3) | |
| One | 4.45 Gy | 6.6 Gy |
| Two | 3.35 Gy | 4.3 Gy |
| Cumulative | 7.8 Gy | 10.9 Gy |
Fig. 2
Axial planning ultrasound plane from fraction 1 with prescribed dose being 12.6 Gy. Image shows gross tumor volume (orange), planning tumor volume (red), prostate (blue), and urethra (green), with isodose doses distributions of 130% (white) and 100% (yellow). Rectal balloon spacer implant (RBI) is marked green between prostate (light blue) and rectum (dark blue)
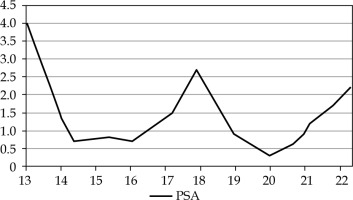
The treatment was tolerated well, with no acute rectal toxicity and no further toxicities reported up to 4 years post-treatment. Furthermore, the patient reported only moderate urinary tract obstruction described as grade 1 according to the CTCAE, version 4.03 [17]. At 18 months after salvage HDR, the PSA dropped to 0.3 ng/ml. Most recent PSA value dating December, 2022 was 2.2 ng/ml, which is not PSA-nadir + 2.0 ng/ml, and not strict considered as a biochemical recurrence, however it is slightly indicative (Figure 3).
Discussion
Curative treatment for locally recurrent disease after initial radiation treatment remains difficult and very challenging. This is even more so in the context of significant late toxicity after initial treatment, which leads to the onset of significant late toxicity. The vast majority (i.e., 98%) of the patients in this setting are offered palliative androgen deprivation therapy (ADT). Salvage treatment is mainly hampered by high toxicity risk after previous irradiation. However, life-long ADT is also associated with significant side effects, including hot flashes, erectile dysfunction, and loss of interest in sexual activity, thereby limiting patients’ quality of life, with an increased risk of cardiovascular diseases, neurological deficits, and diabetes resulting in an increased mortality [21-25]. A window of opportunity for limiting the toxicity of salvage treatment may be focal salvage treatment instead of treating the whole gland. In the case of prostate cancer, this concept and rationale behind focal salvage treatment has been elaborately described in previous research [26-30]. In the current paper, we described a favorable oncological and toxicity outcomes with focal salvage HDR after previous rectal toxicity due to LDR treatment. In this context, both the focal treatment and the rectal balloon spacer offered a therapeutic window, through which we were able to deliver an adequate tumor dose while minimizing the rectal dose. Salvage HDR treatment is a relatively new concept, and it has been described previously, amongst others, by Maenhout et al. [31]. They analyzed favorable toxicity profiles of 17 focal patients treated with a single 19 Gy fraction, with only one case of grade 3 toxicity post-salvage HDR after 10 months of follow-up. Another case series from Maenhout et al. among 30 patients and 24 months of follow-up resulted in only 2 patients with a grade 2 and 3 urinary toxicity each [32]. Acute toxicity after salvage HDR treatment is overall limited to grade 1 or 2 with only few cases of grade 3 toxicity (3-14%) [9, 14, 15, 33, 34]. A recent study by Chitmanee et al. reported 25% of grade 2 and 5% of grade 3 genitourinary late toxicities, with 8% of grade 2 gastrointestinal toxicity [33]. Chen et al. demonstrated only 3% of late grade 3 genitourinary and 4% gastrointestinal toxicity [14]. Biochemical-free rate at 3 years ranges from 46% to 76% in whole gland salvage HDR [9, 14, 15, 33]. There is only limited evidence of the local biochemical control after focal salvage treatment. One study by Murgic et al. reported relapse-free rate at 3 years of 61%, which is comparable with whole gland salvage outcomes [16]. In our patient, a complicating factor was the previous grade 3 rectal toxicity and the location of the recurrent disease in close vicinity of the anterior rectal wall. Before treatment, colonoscopy showed no signs of radiation proctitis, but the patient remained at a high-risk of rectal perforation and/or ulceration. In order to decrease rectal dose, the use of a rectum spacer has proven to be effective in decreasing acute and late rectal toxicities [19, 35]. We used a saline-filled RBI, which is composed of biodegradable polymers that biodegrades within the human body within 3-6 months. Previously, the use of a RBI has been proven to be safe and effective, but not without risks [36, 37]. The balloon had evaporated 6 months post-treatment, and no RBI-related toxicity had occurred.
An important limitation of the proposed workaround is that the knowledge of rectum spacers is relatively limited in re-irradiation setting; therefore, more clinical studies are needed to prove its safety in this category of patients. Potential side effects include rectum perforation and/or necrosis, perineal abscess requiring drainage, recto-urethral fistula, proctitis requiring colostomy, and severe urosepsis necessitating intensive care unit level care, which are reported in radiotherapy-naïve prostate-rectum tissue [38]. The implantation of RBI was complicated by sub-mucosal scarring, increased fibrosis, and cicatrization of the surrounding tissues due to previous radiation toxicity, which could theoretically increase the potential side-effects; however, in our case, it was successfully implanted. The advantage of the inflatable RBI system is that it allows for post-implant correction of the RBI position, whereas liquid spacers (hydrogels, human collagen) do not permit any correction once injected [19].
Conclusions
Here, we described a case of successful focal HDR salvage treatment with the use of a RBI after previous grade 3 rectal radiotherapy-related toxicity. At 4 years of follow-up, no treatment-related toxicities occurred, and there is no evidence of biochemical recurrence. At 4 years of follow-up, no treatment-related toxicities occurred, and there is no evidence of biochemical recurrence according Phoenix definition.
In our opinion, this treatment strategy using focal salvage brachytherapy in combination with a RBI could be considered in this specified high-risk feature patients’ population to obtain the best outcomes and survival. However, more research is needed to confirm safety and efficiency in this specific patients’ group.