Purpose
Gel spacer has been used to create a space between clinical target volume (CTV) and organs at risk (OARs) to protect them from toxic high-dose radiation exposure in radiation therapy of prostate cancer or gynecologic malignancies. In a phase III clinical trial, its protective property for rectal toxicities was demonstrated in external beam radiation therapy for prostate cancer. Likewise, our previous reports have shown that the gel spacer could reduce rectal and bladder dose and reduce frequency of late rectal bleeding [1-4]. However, to the best of our knowledge, it has not been used for head and neck radiation therapy. In this case report, we presented a case with previous whole neck irradiation and developed lymph node metastasis in the reconstructed jejunum mesenteric lymph nodes, who received palliative image-guided interstitial brachytherapy (IG-ISBT) to obtain local control. Because this patient already had received 45 Gy of whole neck radiation therapy 24 years ago, and the reconstructed jejunum should be protected due to small bowel’s tolerance dose being relatively low, spacer gel injection was utilized during IG-ISBT to protect these OARs from high-dose radiation exposure (complication probability of 5% after 5 years (TD 5/5) for late toxicity at a dose of 50 Gy for irradiating 1/3 of the small bowel was reported [5]).
Written informed consent was obtained from the patients for spacer gel injection. This case report was approved by the Institutional Review Board of National Cancer Center Hospital, with Number 2017-091, according to ethical standards of the Declaration of Helsinki.
Case description
A 72-year-old male, with a previous history of 45 Gy in 18 fractions of bilateral whole neck radiation therapy (level I-V) performed using a single antero-posterior-oriented cobalt-60 beam irradiation 24 years ago for malignant lymphoma, and suffered from T3N0M0 hypopharyngeal squamous cell carcinoma in 2004. He underwent total pharyngolaryngectomy and bilateral neck dissection with jejunum interposition. Ten years later, the patient experienced bilateral regional lymph node metastasis, including mesenteric lymph node in the reconstructed jejunum, and received total removal of the reconstructed jejunum and metastatic neck lymph nodes with secondary jejunum reconstruction. Three years later, the patient experienced regional lymph node metastasis, including mesenteric lymph node in the secondary reconstructed jejunum, and received total removal of the reconstructed jejunum and metastatic neck lymph nodes with tertiary jejunum reconstruction. Two years later, the patient experienced third-time regional lymph node metastasis, which was confirmed as squamous cell carcinoma by fine-needle aspiration cytology. The patient received six cycles of a combination of 5-fluorouracil/carboplatin/cetuximab (5 FU/CBDCA/C-mab), which resulted in a partial response. However, eight months after systemic chemotherapy initiation, progressive disease was noted, and nivolumab was started. Six months after the start of nivolumab, positron emission tomography-computed tomography (PET-CT) showed recurrent disease in the head and neck region, including bilateral level V and multiple mesenteric lymph nodes involvement (Figure 1). At this point, further surgical treatment was not planned, and systemic chemotherapy composed of a combination of 5 FU/CBDCA/C-mab was started. Six cycles of 5 FU/CBDCA/C-mab resulted in a partial response; however, seven months after the initiation of 5 FU/CBDCA/C-mab, progressive disease was noted and subsequently, nivolumab was started. Five months after the initiation of nivolumab, during its 9th cycle, the patient felt discomfort with persistent mesenteric lymph nodes (Figure 1), and wanted to receive palliative radiation therapy to control metastatic lymph nodes. Although the larynx was previously totally removed because he already received 45 Gy of whole neck irradiation 24 years ago, high-dose-rate interstitial brachytherapy (HDR-ISBT) was selected, trying to confine radiation exposure to OARs surrounding the target volume as little as possible. The prescription dose per fraction was 7 Gy (D90 > 7 Gy), in which 100% isodose line covered the CTV, and irradiation was performed twice daily at 6-hour intervals (Figure 2). A total of 42 Gy in 6 fractions were delivered in 3 days with needle inserted in place for 3 days. Needle insertion was performed under ultrasonography guidance, so that the needles pierced all enlarged nodes, and 5-French ProGuide® sharp plastic needles (Nucletron, an Elekta Company, Elekta AB, Stockholm, Sweden) were used. Because the enlarged mesenteric lymph nodes were located between the reconstructed jejunum in the right side and left carotid artery, a hyaluronic acid gel spacer (Suvenyl®, Chugai Pharmaceutical Co., Tokyo, Japan) was applied to create a distance between high-dose area and OARs, including the reconstructed jejunum and the left carotid artery (Figure 3). CTV D90 was 9.0 Gy and CTV V100 was 99.3%, while the reconstructed jejunum D1cc and left carotid artery D1cc were 2.4 and 3.8 Gy, respectively. The gel spacer injection was performed once per day in the morning fraction under ultrasound guidance. No acute radiation-induced side effect was noted, apart from grade 1 dermatitis. PET-CT performed three months after the completion of HDR-ISBT resulted in a complete metabolic response (Figure 4).
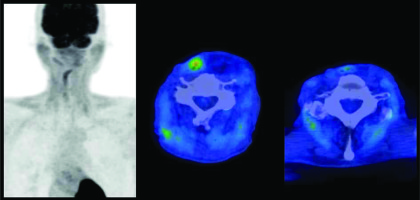
Fig. 1
Fluorodeoxyglucose (FDG)-PET-CT images before salvage high-dose-rate interstitial brachytherapy for mesenteric lymph nodes of reconstructed jejunum. Red color represents high, and blue color represents low uptake on the color scale
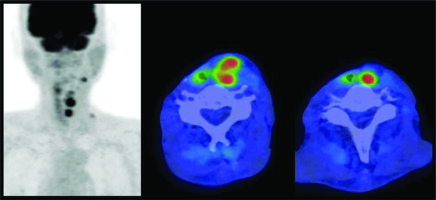
Fig. 2
Dose distribution of high-dose-rate interstitial brachytherapy. Blue, red, green, and sky-blue lines represent 200%, 100%, 80%, and 50% isodose lines, respectively. The light blue object on the right neck represents reconstructed jejunum, yellow circles show carotid arteries, and red dotted line indicate clinical target volume
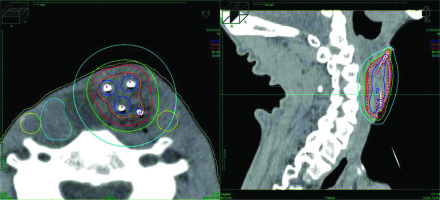
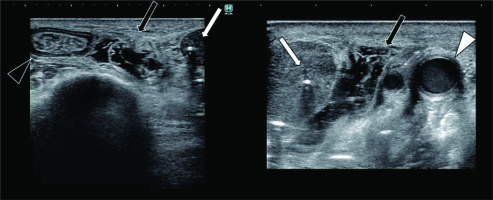
Fig. 3
Axial ultrasonography images. Needle insertion was performed under ultrasonography guidance. Four needles (ProGuide® sharp plastic needle, Nucletron, an Elekta Company, Elekta AB, Stockholm, Sweden) were inserted to irradiate metastatic mesenteric lymph nodes. White arrow represents metastatic mesenteric lymph node, black arrow indicates gel spacer, white arrowhead shows left carotid artery, and black arrowhead represents reconstructed jejunum
Discussion
Gel spacer has been utilized to create a space between CTV and OARs in both external beam radiation therapy (EBRT) and brachytherapy (BRT) [1-4, 6-9]. In a phase III clinical trial, it was demonstrated that SpaceOARTM (Augmenix, Inc., Waltham, MA, USA), polyethylene-glycol hydrogel spacer could reduce rectal dose and rectal toxicity [8]. Our group has been using hyaluronate spacer gel SuvenylTM (Chugai/Roche, Tokyo, Japan) for gynecologic BRT, and reported its usefulness in reducing rectal dose [1], bladder dose [2], and frequency of rectal bleeding [4]. By physically creating a space between CTV and OARs, it is possible to deliver adequate tumoricidal dose to CTV, while reducing doses to OARs. Moreover, it is possible to raise the likelihood of cure by widening a therapeutic window of tumor control.
The head and neck region is also the appropriate anatomical area for applying BRT, either definitive or in combination with external beam radiation therapy [10-21]. However, unlike pelvic organs, only a small amount of soft tissue is available in the head and neck region. Therefore, it is less likely that the gel spacer is utilized for organ protection in head and neck BT. Kishi et al. previously reported that gel spacer could be used to shift critical OARs from surrounding target volume in re-irradiation settings for 30 patients after full-dose radiation therapy, including 13 head and neck cancer patients [6]. In line with this report, gel spacer effectively shifted critical OARs in the current case. The prescription dose of 7 Gy per fraction was selected because it was anticipated that gel spacer could push the reconstructed jejunum and carotid artery away from high-dose area. Dose per fraction lower than 4 Gy could be considered, but since there were so many patients who needed BRT, there was no room for multiple treatment sessions for this patient. Therefore, a short treatment schedule was selected due to logistic reasons.
The limitations of this report is that there was no CT without gel spacer; accordingly, there was no direct comparison of OARs’ doses between those with and without gel spacer. However, due to BRT’s nature of steep dose fall-off close to the sources, it was evident that by simply creating a small gap between a radioactive source and OARs, it will reduce OARs’ radiation exposure considerably. Additionally, only dosimetric results were shown, and no long-term clinical consequence was provided in this report. Therefore, further follow-up and more cases with similar clinical scenarios are mandatory to obtain long-term clinical advantages of this technique. To the best of our knowledge, this was the first report on applying gel spacer to protect the reconstructed jejunum after total laryngectomy. Due to the complex anatomy of the head and neck region, with only a small amount of fat and soft tissues surrounding OARs, it was generally difficult to apply gel spacer to protect OARs. However, when applicable, gel spacer plays a vital role in delivering tumoricidal dose, while decreasing doses to OARs’ to prevent late radiation-related toxicities. Further studies are needed to establish the role of gel spacer in managing head and neck malignancies.
Funding
This work was in part financially supported by the Japan Agency for Medical Research and Development (AMED) and the National Cancer Center Research and Development Fund (26-A-18 and 26-A-28).
Consent for publication and ethics approval and consent to participate
We obtained a written informed consent from the patient for publication including each clinical datum as images, case history, and other clinical data. This case report was approved by the Institutional Review Board of the National Cancer Center Hospital (approval Number, 2017–091), according to the ethical standards laid down in the Declaration of Helsinki.