Purpose
Whole breast radiotherapy (WBRT) is a standard treatment method following breast conserving surgery (BCS) that has been shown to reduce the local recurrence rate in early-stage breast cancer cases [1-3]. Accelerated partial breast irradiation (APBI) has been suggested as a viable alternative to WBRT with high-dose-rate (HDR) multicatheter interstitial brachytherapy (MIB), providing more randomized data available than many other modalities of APBI [4, 5]. The 2018 American Brachytherapy Society (ABS) consensus statement [6] strongly recommends MIB as APBI technique based on two randomized trials with mature follow-up results, demonstrating equivalent rates of local control (LC) and overall survival (OS) compared with WBRT in early-stage breast cancer [7, 8]. Polgár et al. reported results of a phase III clinical trial, where 20-year LC rates at a median 17-year follow-up were 90.4% and 92.1% for 128 MIB and 130 WBRT patients, respectively [7]. Strnad et al. published results of a phase III Groupe Européen de Curiethérapie (GEC-ESTRO) multicenter trial, in which the five-year local control rates at a median 6.6-year follow-up were 98.6% and 99.1% for 633 MIB and 551 WBRT patients, respectively [8].
Although there have been several non-randomized MIB trials, no randomized trials have yet been conducted on MIB in Japan. The first non-randomized MIB-APBI trial started in 1998, and showed promising preliminary results [9]. This trial used the open cavity implantation technique during BCS, and MIB was shown as a suitable treatment method for smaller breast size Japanese women. The dose-fraction schedule was 36 to 42 Gy in six to seven fractions, which generated a LC rate of 95% at a median follow-up of 52 months without excessive adverse events. A subsequent trial was conducted at National Hospital Organization Osaka National Hospital in 2002 using a similar protocol achieving a LC rate of 96%, thus confirming the results reported in the former study [10]. However, the complication rate was higher, with overall 16% of wound complications and 4% of rib fractures. Based on these preliminary results, we conducted the first multi-institutional prospective study in Japan using 36 Gy in six fractions [11], with a LC rate of 100% at a short-term follow-up of median 42 months [12]. At present, the longest follow-up MIB results in Japan were reported by Sato et al. The authors reported on 516 MIB patients using a dose-fraction schedule of 32 Gy in eight fractions, achieving a LC rate of 97.5% with a median follow-up of 53.1 months [13]. In order to confirm these data, we updated in this report our oncological outcomes on 45 MIB patients extending the median follow-up from 31 months [10] to 10 years.
Material and methods
APBI treatment and management policy
This research project was approved by the local institutional review board. Our treatment technique evolved over three periods while encompassing patients with the following inclusion criteria: 1. Invasive or non-invasive ductal or invasive lobular carcinoma without distant metastases; 2. Patient’s age < 80 years without a history of collagen vascular diseases or prior thoracic irradiation; and 3. ECOG performance status < 3.
During the first period from June 2002 to June 2003, we performed APBI on three patients as a pilot study. All three patients were treated using the open cavity implantation technique, receiving a dose of 36 Gy in six fractions. Clinical target volume (CTV) was defined as the excisional cavity plus a margin of 3 cm. Prophylactic antibiotics were applied throughout the entire treatment period until the completion of MIB. Based on the results obtained within this pilot setting, 20 patients were treated in the second period as part of phase I/II studies. starting in June 2003 (local institutional review board trial number, 0329). CTV was defined as in period one based on open cavity surgery; however, prophylactic antibiotics were administered only during applicator implantation and not during irradiation period. This approach was discontinued after 23 patients due to an unexpectedly high-rate of wound complications (with or without infection) in 7 patients.
Under consideration of these findings, we modified our protocol in the third period, which was initiated in October 2004. As a part of our adaptions, we re-introduced the use of prophylactic antibiotics throughout the entire treatment. Although no clinical data showing the importance of the use of prophylactic antibiotics have been published, some reports have observed the usage of prophylactic antibiotics at that time [14-18]. Therefore, we changed our protocol. Moreover, we excluded patients with > 4 axillary lymph node metastases because new evidence was prevalent [19]. In addition, CTV was reduced by limiting the safety margin around the excisional cavity at 2 cm compared with initial 3 cm, according to the RTOG 9517 protocol. With that protocol, 54 patients were treated using the closed cavity technique (with implantation being performed after complete wound healing following BCS), and nine patients were treated using the open cavity technique.
During the third period, the patients with tumor size larger than 3 cm, history of neoadjuvant chemotherapy, positive surgical margin, and age less than 40 years old were excluded according to Groupe Européen de Curie-thérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) 2009 recommendations [20] and Japanese prospective multi-institutional feasibility study protocol [11] since 2010.
Applicator implantation
Our open cavity technique has been described elsewhere [10]. In short, open cavity applicator implantation commenced immediately after BCS. Oriented by at least four surgical clips, which were placed intra-operatively to enable demarcation of the excisional cavity, open-end metal trocar needles of 200 mm in length were implanted under clinical guidance to define three dimensional implant geometry, and were subsequently replaced by flexible afterloading catheters for HDR irradiation (single-leader flexible implant tube and Oncosmart catheter system®, Nucletron, ELEKTA AB, Sweden). In contrary, closed cavity implantation was performed after complete wound healing, following BCS. In analogy to the open cavity technique, metal trocar needles were implanted under ultrasonography guidance to define multiplane implant geometry, and similarly, subsequently replaced by flexible afterloading catheters. We took care to implant the superficial plane at 7-8 mm under the skin surface to ensure adequate catheter contribution for CTV coverage while avoiding high-dose skin levels. In patients undergoing the open cavity technique, treatment commenced four to five days after implantation, whereas in the closed cavity protocol, within 48 hours after implantation.
Dose planning and treatment
Following applicator implantation, treatment planning was performed. For the first 24 patients, planning was X-ray-based with superimposition of the additional CT data set. For the remaining 62 patients, anatomy-oriented planning was exclusively CT-based. 3D dose optimization was performed using PLATO® or Oncentra Brachy® (Nucletron, ELEKTA AB, Sweden) [21]. Planning target volume (PTV) was defined as CTV excluding the nearest subcutaneous tissue (5 mm from the skin surface). Seventy-eight patients received 36 Gy in six fractions over three days, and eight patients with close/positive surgical margins were treated with 42 Gy in seven fractions over four days. Margin status was evaluated using Japanese 5 mm pathological method [22]. These dose-fraction schedules were defined according to first Japanese clinical trial initiated since 1998 [9]. These schedules were equal to 48 Gy and 56 Gy of equivalent dose in 2 Gy fraction (EQD2) (α/β = 10), because these doses were similar to standard tangential radiotherapy (50 Gy and 60 Gy).
Our planning goals were V100 (volume receiving 100% of the prescribed dose) ≥ 90% with a dose non-uniformity ratio [volume receiving 150% of the prescribed dose (V150) divided by V100, DNR] ≤ 0.35. Maximum point dose of the skin was set to be ≤ 90% of the prescribed reference dose. Ninety percent of 36 Gy in 6 fractions and 42 Gy in 7 fractions were equal to 54 Gy and 64 Gy of EQD2 (α/β = 3), respectively. These doses were also similar to standard tangential radiotherapy (50 Gy and 60 Gy). In all cases, treatments were performed using an iridium-192 HDR afterloading system (microSelectron-HDR®, Nucletron, ELEKTA AB, Sweden). All patients were treated after signing a written informed consent.
Analysis
Primary endpoint was local control rate, and secondary endpoint was late complication. For the current analysis, the patient’s sample was deduced from our prospectively maintained database and retrospectively analyzed. Using Kaplan-Meier method, the likelihood of events was deduced and thereafter compared using χ-square test. A two-sided p-value of < 0.05 was set as statistically significant. Complications were evaluated using common terminology criteria for adverse events, version 4.0.
To analyze the treatment outcome according to the European and US risk stratifications, 2009 GEC-ESTRO risk stratification [20] and 2018 ABS risk stratification [6] were used. To re-classify our patients according to these stratifications accurately, an estimation was made whether the surgical margin status was clear within 2 mm or not. However, it was impossible because Japanese pathologists did not use an inked margin technique. Japanese pathologist sliced the surgical mammary gland materials in 5 mm intervals, and all the slices were examined microscopically [22]. Pathological tumor mapping in all slices was made, and evaluated the margin status. Therefore, we could estimate < 2 mm or 2 mm margin status for horizontal direction of pathological specimen. Skin direction and pectoral muscle direction were also assessed. However, we could only estimate < 5 mm or 5 mm for vertical direction because specimen had a 5 mm thickness. Six patients were unclassified by this limitation.
Results
Patient characteristics
Eighty-six patients were treated between 2002 and 2011. Patients’ characteristics are shown in Table 1. The median age was 48 years (range, 26-73 years), and the median follow-up was 119 months (range, 13-189 months). All patients underwent BCS for histologically proven adenocarcinoma. Eighty patients were diagnosed with invasive ductal carcinoma, and the remaining six patients with ductal carcinoma in situ. The 2002 Union for International Cancer Control (UICC) classification was applied for T-stage classification. Two patients were classified as pT0, six patients as Tis, 55 patients as pT1, 22 patients as pT2, and one patient as pT3 after BCS. The surgical resection margins were re-classified as adequate (≥ 2 mm) in 68 patients, and as close/positive margin (< 2 mm) in 12 patients. Six patients were unclassified. Axillary lymph node metastases were found in 16 patients with APBI treatment of pN2 patients being terminated in 2004, and of pN1 patients in 2010 in consideration of breast cancer treatment policy changes. Fourteen patients received neoadjuvant systemic treatment. Of those, two were treated with hormone therapy, ten with chemotherapy, and two with both treatments. Seventy-four patients received adjuvant therapy. Of those, 49 were treated with hormone therapy, eight with chemotherapy, and 14 with both treatments. Three patients received a molecularly targeted drug (trastuzumab).
Table 1
Patients’ characteristics
Dosimetry
A median of 12 interstitial applicators (range, 6-18 applicators) were used in order to generate two or three plane implants covering a median PTV of 45.7 cc (range, 10.3-215.4 cc). The median V100, V150, and V200 (volume receiving 200% of the prescribed dose) were 188.6 cc (range, 73.0-315.1 cc), 51.7 cc (range, 19.8-82.6 cc), and 19.2 cc (range, 9.0-31.1 cc), respectively. The median DNR was 0.30 (range, 0.2-0.59). Nine of the 86 patients (10%) had a DNR > 0.35.
The median maximum skin surface dose (skin point dose) per fraction was 4.8 Gy (range, 3.3-15.0 Gy), with a median total maximum skin surface dose of 29.4 Gy (range, 19.8-90.0 Gy). The median maximum lung surface dose (lung point dose) per fraction was 4.2 Gy (range, 2.0-6.3 Gy), with a median total maximum lung surface dose of 25.8 Gy (range, 12.0-44.1 Gy).
Treatment outcomes
Treatments were completed in all patients without post-interventional complications needing therapy, interruptions due to acute radiogenic adverse events, or technical issues affecting treatment fraction execution.
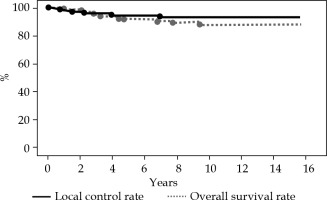
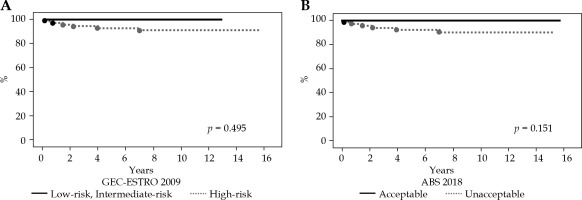
At a median follow-up of 119 months (range, 13-189 months), the 5- and 10-year LC rates for the entire cohort were 94% and 93%, respectively (Figure 1). The 5- and 10-year OS rates for the entire cohort were 92% and 88%, respectively. Overall, six local recurrences occurred at 2, 9, 18, 27, 48, and 84 months after MIB, respectively. All recurrent lesions were actual infield recurrences or in the low-dose spillage volume around PTV. When classifying our patients according to the 2009 GEC-ESTRO risk stratification scheme, six patients were classified as low-risk, 9 as intermediate-risk, and 69 as high-risk patients for APBI. Two cases were unclassified because we could not define the surgical margin status. The 10-year LC rate was 100%, 100%, and 91% for low-risk, intermediate-risk, and high-risk patients, respectively (Figure 2A). In relation to the 2018 American Brachytherapy Society (ABS) risk stratification scheme, 21 patients were classified into the APBI acceptable group, and 63 into the APBI unacceptable group. Two patients were unclassified because we could not define the surgical margin status. The 10-year LC rate was 100% and 90% for acceptable and unacceptable patients, respectively (Figure 2B).
Fig. 2
A) Kaplan-Meier curve for actuarial local control rates based on the 2009 Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) risk stratification. B) Kaplan-Meier curve for actuarial local control rates based on the 2018 American Brachytherapy Society (ABS) risk stratification
We excluded patients with > 4 axillary lymph node metastases, tumor size larger than 3 cm, history of neoadjuvant chemotherapy, positive surgical margin, and age less than 40 years old since 2004 or 2010. Until exclusion, 36 patients were already treated. The-10-year LC rate was 89%.
Distant metastasis was documented in 13 patients (15%), with no cases of exclusive regional failure in the entire cohort. Ten patients died so far. In eight cases, the cause of death was breast cancer, and in the remaining two intercurrent diseases.
Acute complications
No case of ≥ grade 3 dermatitis was observed, and no patient required analgetic treatment for < grade 2 skin reactions. Wound complications were observed in 7 patients (8%), all of whom were treated during the second period of our protocol evolution with open cavity surgery, and prophylactic antibiotics only during applicator implantation but not during fractionated HDR irradiation. Of those, three patients required wound re-opening and drainage for symptomatic infection. The remaining seven patients were treated conservatively. No wound complications were observed in any of the patients who received prophylactic antibiotics throughout MIB and underwent closed cavity implantation. Risk factors for wound complications were omission of prophylactic anti-- biotics during MIB, open cavity implantation, and larger V100 values (Table 2).
Table 2
Comparison of background between wound trouble (+) and (–) groups
Late complications
No grade 3 or higher skin reactions were observed. Symptomatic fat necrosis was noted in two patients (2%). It occurred at 15 and 32 months after MIB. Both the patients showed cystic induration. One patient complained of slight pain without any medication intervention.
The dosimetric/volumetric indices of the first patient were V100, V150, V200, and DNR of 211.9 cc, 51.9 cc, 25.8 cc, and 0.24, respectively. In the second case, the respective values for V100, V150, V200, and DNR were 146.7 cc, 40.3 cc, 15.2 cc, and 0.27. Both the patients did not require invasive treatment. No symptomatic radiation pneumonitis was observed within eight patients (9%) with rib pain. In all 8 patients, CT or bone scintigraphy revealed minor rib fracture at implantation level. All cases symptoms had resolved at the time of the latest follow-up.
Discussion
Accelerated partial breast irradiation using MIB is an established treatment method for adjuvant radiotherapy (RT) in early-stage breast cancer [6-8, 20, 23, 24]. In addition, MIB has been shown effective and safe for adjuvant APBI after second BCS in patients with previous WBRT [25]. Multiple studies with long-term follow-up exceeding nine years reported 10-year LC rates of 90-99%, with OS rates of 72-85% [26-30] (Table 3). We started MIB, and the median follow-up time was 119 months for 86 patients. A number of studies from Japan advocated against the use of MIB for APBI [9-13]. In Japan, this may be due to the high prevalence of young breast cancer patients with relatively small cup sizes [31]. There are several authors reporting from the Asia area [9-13, 29, 32, 33]. Budrukkar et al. reported on 239 Indian patients treated with MIB for APBI [29]. With a median follow-up of 114 months, they analyzed their results in relation to international consensus statements using various classification systems. According to the 2009 GEC-ESTRO risk stratification scheme [16], their LC rates were 89%, 94%, and 87%, for low-risk, intermediate-risk, and high-risk patients, respectively. In relation to the 2018 ABS consensus statement [6], LC was 91% and 89% for acceptable and unacceptable patients, respectively. In our series, the respective 10-year LC rates were both 100% for low-risk and intermediate-risk according to the 2009 GEC-ESTRO risk stratification scheme.
Table 3
Treatment results of institutes that reported long-term follow-up results
| Hungary [7] | RTOG 9517 [26] | Washington University [27] | St. Maria Hospital [28] | Tata Memorial Hospital [30] | William Beaumont Hospital [29] | Present study | |
|---|---|---|---|---|---|---|---|
| Number of patients | 128 | 98 | 175 | 133 | 240 | 199 | 86 |
| Median follow-up (years) | 17 | 12.1 | 10 | 9.2 | 9.5 | 9.6 | 9.9 |
| Dose/ fractions | 36.4 Gy/7 fr. | 34 Gy/10 fr. | 34 Gy/10 fr. | 32 Gy/8 fr. | 34 Gy/10 fr. | 32 Gy/8 fr. | 36-42 Gy/6-7 fr. |
| Skin | 13.6% (grade 2-3) | N.A. | 0.6% (grade 3), 25% (grade 1-2) | 4% (grade 2) | N.A. | N.A. | 2% (grade 2) |
| Others | N.A. | N.A. | N.A. | 2% (fat necrosis), 9% (rib pain) | |||
| 10-year local control rate | 94% | 94% | 92% | 99% | 90% | 95% | 93% |
| 10-year overall survival rate | 77% | 81% | 85% | 84% | 72% | 88% |
From Japan, Sato et al. compared the results of MIB with the outcomes of WBRT as adjuvant treatment following BCS, focusing particularly on patients’ age [34]. Of overall 184 patients, 99 received MIB and 85 WBRT, with patients’ age ranging from 30 to 49 years. The 4-year LC rate was 97.0% for MIB and 97.6% for WBRT, with a 4-year disease-free survival of 97.6% for MIB and 91.4% for WBRT. There was no significant difference between the two groups in terms of disease control and toxicity along all age sub-groups. However, it does not mean that all high-risk patients should be candidates for APBI, because our treatment results for high-risk (91%) and unacceptable (90%) groups tended to be worse than the other groups. Especially, in the-10-year LC rate of 36 patients with > 4 axillary lymph node metastases, tumor size larger than 3 cm, history of neoadjuvant chemotherapy, positive surgical margin, and age less than 40 years old, the rate was 89%. We considered that we must carefully compare the treatment outcome with WBRT to decide the adequate eligibility criteria for Asian woman. At present, our results could only suggest that MIB is an effective treatment option for intermediate-risk patients.
The acute complication rate observed in our study is comparable with profiles reported by other authors. Wound complications were observed in 7 patients (8%), all of whom were treated during the second period of our protocol evolution with open cavity technique. Chen et al. [35] reported on 199 patients with 22 (11%) experiencing symptomatic wound complications. Of those, 14 patients showed an acute infection and eight a delayed tumor bed infection, with 17 of 22 (77%) having been treated with the open implant technique. These data corroborate with our observation that omission of prophylactic antibiotics during treatment and open cavity implantation may be risk factors for wound complications. However, more data are necessary.
Concerning late skin toxicity, this study showed tolerable results (grade 2, 2%) compared with other institutes that reported long-term follow-up outcomes (Table 3). In a large study, Polgár et al. investigated the late complication rate in their GEC-ESTRO trial among 655 patients treated with MIB for adjuvant APBI [36]. No ≥ grade 4 complications were observed, with grade 3 skin reactions documented in only three patients (< 1%). In the current study, no ≥ grade 3 late skin adverse events were noted. Our attempt to reduce skin and lung point dose to < 90% and < 100% of the prescribed dose, respectively, might have contributed to this result. On the other hand, fat necrosis (2%) and minor rib fracture (9%) were seen as non-skin toxicity. At this, Wazer et al. described V150 and V200 as significant risk factors for fat necrosis reporting a V150 of 69 ±11.9 cc and V200 of 22 ±3.3 cc for patients experiencing this complication [37]. In our study, one patient violated this critical dosimetric value with a calculated V200 > 22 cc. Likewise, Ott et al. [38] showed a correlation between V200 and fat necrosis after conserving surgery and APBI in women with breast cancer.
Our results suggest MIB as a safe and effective treatment option for adjuvant APBI even in intermediate-risk Japanese patients. However, there are still few articles from Asian countries. Multi-institutional and multinational studies are desirable.