Purpose
Prostate cancer is the most common solid tumor in men [1]. With the introduction of serum prostate-specific antigen (PSA) testing, the incidence of prostate cancer has increased in recent years [2]. Changes in screening recommendations indicate that about 90% of patients are diagnosed with localized prostate cancer (LPC) [3].
There are different modalities for radical treatment of LPC, including radical prostatectomy, external beam radiotherapy (EBRT), and brachytherapy (BT) [4].
Brachytherapy treatment can be delivered by high-dose-rate (HDR) or low-dose-rate (LDR), and can deliver a higher radiation dose to the target, avoiding surrounding tissues. The use of high doses per fraction has a biological dose advantage for tumors with a low α/β ratio, such as prostate cancer. A higher dose per fraction regimen leads to an increased tumor control. Due to these characteristics, BT is commonly the primary treatment for LPC and achieves excellent outcomes in terms of disease control and toxicities [5,6,7].
High-dose-rate BT has various technical advantages over LDR. For example, needle catheters can be placed outside of the prostate gland, which allows for improved coverage in cases of extracapsular extension or seminal vesicle invasion. Moreover, by using HDR-BT techniques, radioactive source dwell-time positions can be programmed directly by the physician. This increases the dose to tumor area and reduces the dose to organs at risk, including the urethra and rectum [8,9].
Different studies have reported the results of HDR-BT as a monotherapy for the treatment of LPC, confirming its efficacy and low toxicity [10,11,12,13,14,15,16,17,18,19,20].
While the use of brachytherapy in prostate cancer is well-known, currently, the total dose and fractionation for HDR-BT in the treatment of localized prostate cancer are not yet established, and finding the optimal HDR brachytherapy schedule remains a challenge.
Our study aimed to retrospectively evaluate clinical outcomes in patients affected by LPC and treated with 3D conformal HDR-BT as monotherapy.
Material and methods
Patients and tumor characteristics
From March 2004 to November 2018, a total of 277 men with localized prostate cancer (cT1c-T2cN0M0) were treated in our institute using HDR-BT and 192Ir source.
The data were collected retrospectively by a radiation oncologist. Pre-treatment evaluation included clinical examination, digital rectal examination (DRE), routine pre-operative determination of blood chemistries and blood counts, PSA, Gleason grade, and computed tomography (CT) or magnetic resonance imaging (MRI) in selected cases.
After HDR-BT treatment, all patients continued regular follow-up in our institution, with clinical examination, DRE, biochemical control, and additional radiologic tests if clinically indicated. Gastrointestinal and urinary toxicities was evaluated at the clinical examination according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 5 of 2017.
All patients, after histologically confirmed prostate cancer, were stratified into low-risk prostate cancer (stage T1-T2a, Gleason score [GS] ≤ 6, and PSA ≤ 10), intermediate-risk prostate cancer (stage T2b-T2c and/or GS = 7, and/or PSA > 10-20), and high-risk prostate cancer (stage > T2c and/or GS = 8-10, and/or PSA > 20) according to D’Amico et al. and the EAU criteria [21,22]. The median age was 67 (range, 47-81) years.
Of them, the majority of patients (94.6%) were low- and intermediate-risk (145 patients were low-risk and 116 intermediate-risk), and only 5.7% resulted high-risk (16 patients). Overall, neoadjuvant androgen deprivation was administered in 33.9% of patients for a median of 3 months. Finally, only 2.2% of patients received adjuvant androgen deprivation therapy (ADT). Patients and tumor characteristics are shown in Table 1.
Table 1
Patient and tumor characteristics
HDR-BT technique
After given informed consent, the patients were placed in a lithotomy position and underwent epidural anesthesia. A urinary catheter was placed into urinary bladder. A 7.5-MHz biplanar transrectal ultrasound (TRUS) transducer was inserted into the rectum to identify the prostate and urethra. The applicators were inserted transperineally under direct ultrasound monitoring control. After insertion and immobilization of anchor needles, the flexible applicator needles were implanted from the anterior to the posterior rows in the periphery of prostate to minimize rotation. Subsequently, the remaining needles for the internal regions were implanted. The treatment plan was done using Oncentra Prostate software to perform 3D conformal dose planning supported by an inverse planning algorithm. Clinical target volume (CTV) was defined by the whole prostate with a 3 mm margin. If seminal vesicle invasion was observed or diagnosed by imaging, the applicator needles were placed with the seminal in the CTV. The planning target volume was defined as equal to the CTV. Dosimetric goals included plaining aim dose to the target was dose (D90) 90% > 95%, dose received by 2cc rectum (D2cc) < 75% of prescription dose (PD), and D2cc bladder < 80% of prescribed dose. Finally, urethra dosimetric goals included the dose received by 1% volume (V1) < 115% PD and 10% volume (V10) < 110% PD.
According to NCCN [4] and ABS guidelines [7], following the evolution of knowledge in HDR-BT as well as usage of a new regimen of fractionation published in the literature and our institutional experience, the total doses were prescribed as follow: 38 Gy in four fractions in 149 patients (period 2004-2010), 27 Gy in two fractions in 41 patients (period 2010-2013), and 19-20 Gy in single fraction in 87 patients (period 2014-2017). In patients receiving 38 Gy, the four fractions were delivered twice daily, with a minimum interval of 6 hours, while in patients receiving 27 Gy, the two fractions were delivered with an interval of 1 or 2 weeks. The treatment was performed using the MicroSelectron® digital HDR (Elekta AB v2).
Statistical analyses
Before performing statistical analysis, an exploration phase was carried out; categorical data were described by frequency, whereas continuous data by a mean and median.
Biochemical progression was defined as post-treatment PSA greater than 2 units above the nadir value. Progression-free survival (PFS) was defined as the time from the HDR-BT treatment to the date of occurrence of any of the following events: local recurrence, local treatment (surgery, re-irradiation), or identification of distant metastasis.
Survival functions and disease control were calculated using the Kaplan-Meier method, and the log-rank test was used to evaluate the differences between curves.
Overall survival was calculated from the date of HDR-BT treatment to the date of death. Cancer-specific survival was defined as the interval of time from BT treatment to the date of cancer-related death.
Univariate analysis was performed including each risk factor in a Cox regression model. The results of the Cox regression were expressed by hazard ratios (HR), with its related confidence interval (CI) and related p-value calculated using a Wald test. Differences were considered significant at p < 0.05. Analyses were performed with a SPSS 22 software data analyses.
Results
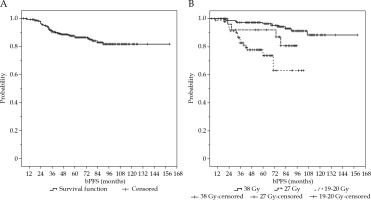
After a median follow-up period of 6 years (range, 6-160 months), the biochemical progression-free survival (bPFS) and PFS were 81% and 96%, respectively. After 3, 5, and 8 years, the bPFS rates were 92%, 85%, and 81%, respectively. Figure 1A and B shows the Kaplan-Meier curves regarding bPFS.
Fig. 1
Kaplan-Meier curves of biochemical progression-free survival A) in all patients and B) in patients treated with 19-20 Gy in single fraction, 27 in two fractions, and 38 Gy in four fractions
Data analyses showed that the median initial prostate specific antigen (iPSA) was 7.85 ng/ml, ranging from 1.8 to 59.5 ng/ml.
Subgroup analysis based on dose prescription showed that in patients treated with a total dose of 38 Gy in four fractions, the bPFS was 91%. In patients receiving 27 Gy in two fractions, the bPFS was 86%, whilst in patients treated with 19-20 Gy in a single fraction, the bPFS was 65%.
Univariate analysis showed that patients receiving 38 Gy in four fractions or 27 Gy in two fractions had a statically significant advantage in terms of bPFS (Figure 2B) compared to those treated with a dose of 19-20 Gy in single fraction (p-value = 0.001, HR = 1.821, 95% CI = 1.239-2.379). There was no statistically significant difference between patients receiving 38 Gy in four fractions compared to patients treated with 27 Gy in two fractions (p-value > 0.05)
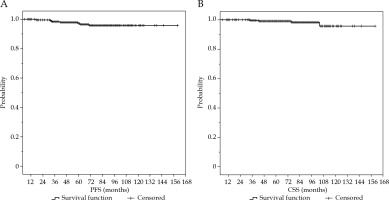
Fig. 2
Kaplan-Meier curves of progression-free survival (A) and cancer-specific survival of all patients analyzed (B)
As expected, the bPFS was higher in patients classified as low- to intermediate-risk prostate cancer compared to patients classified as high-risk prostate cancer (bPFS rate of 90% and 76%, respectively). This difference was statistically significant in favor of patients with low- to intermediate-risk prostate cancer (p-value < 0.05).
Subgroup analyses revealed that this advantage was maintained for patients receiving 38 Gy in four fractions and 27 Gy in two fractions, but was lost in patients treated with a total dose of 19-20 Gy in a single fraction. This finding can be explained by a higher number of biochemical recurrences in this subset of patients regardless of their prostate cancer risk. Moreover, patients with iPSA < 9.4 ng/ml had an advantage in terms of bPFS compared to patients with iPSA ≥ 9.5 ng/ml (p = 0.022, HR = 2.042, 95% CI = 1.123-4.081).
In a subgroup analysis, this advantage was lost in patients receiving 38 Gy in four fractions or 27 Gy in two fractions, and was confirmed in patients treated with a total dose of 19-20 Gy in a single fraction (p-value = 0.0001).
There was no statistically significant difference between patients receiving ADT therapy or not, even in the subgroup analysis of prescribed dose (p-value > 0.05).
Multivariate analysis (Table 2) showed that the total prescription dose and iPSA > 9 ng/ml maintained statistically significant differences and prognostic factors were confirmed for bPFS (p-value < 0.05).
Table 2
Univariate and multivariate analysis of prognostic factors of biochemical progression-free survival in patients affected by localized prostate cancer treated with HDR-BT as monotherapy
Of 42 patients with recurrences, the majority of patients had loco-regional recurrences (85%) and only 15% had distant metastases. Of them, 26 patients (62%) received salvage ADT, eight patients (19%) received EBRT treatment, four patients (9.5%) underwent salvage surgery, and four patients (9.5%) did not receive any salvage therapy, only PSA monitoring.
Overall survival (OS) and cancer-specific survival (CSS) rates were 83% and 97%, respectively. For survival rate, there was no statistically significant difference between patients treated with 19-20 Gy in a single fraction, 27 Gy in two fractions, and 38 Gy in four fractions.
Finally, overall genitourinary (GU) and gastrointestinal (GI) acute toxicities G2-G3 were 28%. Late G2-G3 GU and GI toxicities were very low (2.2%) and of these patients, only three reported G3 late toxicity (0.8%), which involved GU toxicity.
Discussion
The challenge in radiation oncology is to deliver high doses of radiation to the tumor while limiting the dose to surrounding tissue, thereby reducing the risk of toxicity.
It is well-known that a higher dose to the tumor or high-dose per fraction improve the outcomes of radiation therapy in prostate cancer, with acceptable toxicity. Indeed, randomized trials and meta-analyses have shown that a total dose of 78-80 Gy given in a standard regimen and EBRT technique improve a biochemical control for all risk groups [23,24,25,26,27,28,29,30,31].
HDR-BT is commonly used in combination with EBRT or as monotherapy for the treatment of prostate cancer, with excellent outcomes in terms of disease control and toxicities [30,31,32,33,34,35,36,37,38,39,40].
In a comprehensive comparative meta-analysis, Grimm et al. demonstrated that across all risk groups, BT was associated with better bPFS than surgery or EBRT alone [31]. Currently, the total dose and fractionation for HDR-BT in the treatment of LPC are not yet established, and finding the optimal HDR brachytherapy schedule remains a challenge.
For this reason, the American Brachytherapy Society does not recommend a specific dose for fraction schedules [32].
Earlier studies used many small fractions (often six or more) to prevent possible acute or late toxicity of large doses per fraction [15]. Long-term biochemical control rates of over 90% (with low-rate toxicity < 5%) were reported in patients with an intermediate-risk disease using four-six fraction regimens (6.5-7.5 Gy per fraction) [33].
Zamboglou et al. [35] published their results of 718 patients with clinically localized prostate cancer, treated with HDR-BT as monotherapy with a total dose of 38 Gy in four fractions. After a median follow-up of 4 years, 3- and 8-year biochemical control was 97% and 94%, respectively. Metastasis-free survival rates were 98% and 97%, respectively. Similar results were described by Jawad et al. [37] for favorable-risk prostate patients, who underwent HDR brachytherapy as monotherapy at the dose of 38 Gy in four fractions, 24 Gy in two fractions, and 27 Gy in two fractions. Minimal grade 3 toxicities were observed, but no grade 4 or higher.
Recently, some authors reported results of a single fraction of 19 Gy for the treatment of localized prostate cancer with discordant data regarding biochemical control [38,39,40].
In a prospective study from 2017, Krauss et al. published the results of 63 patients with intermediate-risk prostate cancer, who were treated with a dose of 19 Gy single fraction using HDR brachytherapy. After a follow-up of 2.9 years, the biochemical control rate was 93%. No grade 3 urinary toxicities were reported [38]. The limitation of this study was a short follow-up (< 3 years).
A higher rate of biochemical failure in patients receiving 19 Gy in single fractions has been described in the literature. In a randomized trial, Morton et al. compared single fraction of 19 Gy to two fractions of 13.5 Gy in patients with low- and intermediate-risk disease. Both treatment regimens were very well tolerated, with an acute retention rate of 2.4% and grade 3 toxicity rate of < 1%. Local recurrence was observed in the single fraction arm only [39].
In our study, after a long follow-up, we found excellent results in terms of bPFS and PFS (82% and 96%, respectively), with a superb CSS rate of 97%, despite including patients with high-risk prostate cancer who were unfit for other treatments or refused to have them.
Patients receiving a total dose of 38 Gy in four fractions or 27 Gy in two fractions had a higher biochemical-free progression (90%) compared with those treated with 19-20 Gy in a single fraction (bPFS 65%).
There was no statistically significant difference between patients treated with a 19-20 Gy single fraction, 27 Gy in two fractions, and 38 Gy in four fractions, regarding PFS and OS.
Our results demonstrate low unacceptable biochemical control rates for patients with localized prostate cancer treated with a single fraction of 19-20 Gy compared to patients receiving 38 Gy in four fractions or 27 Gy in two fractions. We did not identify any dosimetric factor associated with the risk of recurrence, which can be described in terms of cancer radiobiology, such as biological effective dose (BED), re-oxygenation, and phases of the cell cycle.
For HDR prostate brachytherapy, simplified-form BED is not appropriate, and full-form BED (considering intrafraction and interfraction repair, repopulation, and time of irradiation) can be considered [40,41,42].
It is estimated that full-form BED for a total dose of 20 Gy in a single fraction corresponds to only 64-82% of simplified-form BED calculation, and that regimen with more than a fraction simplified-form BED correspond to 90-94% of full-form BED [42].
For this reason, the full-form BED calculation seems to be lower in patients treated with a total dose of 19-20 Gy in a single fraction compared to patients receiving 27 Gy in two fractions and 38 Gy in four fractions. Moreover, hypoxia is commonly present in prostate cancer, and administering more than one fraction supports the improvement of tumor response, while using a single fraction, the re-oxygenation effect is lost.
Finally, by using a single fraction, the re-distribution effect can be lost because a proportion of cells may be in relatively radioresistant phases of the cell cycle, whereas treating with more than one fraction enables the re-distribution into other phases, when the cells are more sensitive [43].
Similar to our findings results were described by Prada et al. [44], where 60 patients with favorable clinical LPC underwent HDR-BT at the dose of 19 Gy in a single fraction. After a median follow-up of 6 years, biochemical control was 66%. The overall and tumor-free survivals were 90% and 88%, respectively.
Moreover, Morton et al. [45] recently published their data of a randomized phase II clinical trial, where 170 patients with LPC were randomized to receive HDR as either a single fraction of 19 Gy or as two fractions of 13.5 Gy, one week apart. After a median follow-up of 60 months, 5-year bPFS and cumulative incidence of local failure was 73.5% and 29%, respectively, in the single fraction arm, and 95% (p = 0.001) and 3% (p < 0.001), respectively, in the two-fraction group. Recurrence was not associated with initial stage, grade group, or risk group. The authors concluded that HDR monotherapy delivered as two fractions of 13.5 Gy was well tolerated, with a high cancer control rate at 5 years. Single fractions of 19-20 Gy as monotherapy were inferior and were not recommended.
Based on our long follow-up results, a single fraction of 19-20 Gy as a monotherapy was not adequate for biochemical local control of LPC.
Limitations of our study include its retrospective nature, lack of data regarding acute and late toxicity, and difficulty in obtaining a complete data, since the study was not randomized, and many patients have been lost to follow-up or died due to other causes.
Overall, an optimal results in terms of bPFS and PFS (81% and 96%, respectively) were found, with an excellent CSS rate (97%) after a long follow-up, despite including patients with high-risk prostate cancer unfit for other treatments or who refused other therapies.
Conclusions
In our experience, HDR-BT as monotherapy is a valid modality for the treatment of LPC in terms of biochemical control, local control, and overall survival.
A total dose of 38 Gy in four fractions or 27 Gy in two fractions, with or without an addition of ADT, was adequate for LPC treatment achieving an excellent biochemical control rate. After a long follow-up, we found lower bPFS in patients receiving 19-20 Gy in single fraction (65%), whereas 19-20 Gy was not adequate for the treatment of localized prostate cancer as a monotherapy and should only be prescribed in selected cases.
HDR-BT was safe and effective, with very low rates of GU and GI acute and late toxicities.
Finally, randomized trials with a longer follow-up are necessary to confirm our results and to define the total dose and dose per fraction for the treatment of LPC with HDR-BT, considering prostate cancer risk classification.



