Purpose
Lip carcinoma has been treated for years with low-dose-rate (LDR) brachytherapy (BT), achieving local control greater than 90%, in both basal and squamous cell carcinomas [1-4]. Functional and cosmetic results are better than those of surgery in medium to large-sized tumors [5]. Currently, only high-dose-rate (HDR) BT is available, yet there are few publications explaining the doses and techniques used in practice. Initial reports with HDR-BT provided satisfactory results [6-8]. Comparison between LDR and HDR showed that they are equivalent in local control, but HDR results in fewer complications [8-11]. Indications for treating lip carcinoma with HDR are described in recommendations for head and neck BT [12]. Pulsed-dose-rate (PDR) BT using plastic tubes or needles has also shown good results [13-16]. Several publications described the use of moulds to treat small lip carcinomas [17-20]; however, the interstitial implantation of rigid needles or plastic tubes is the current standard practice (interventional radiotherapy). The use of parallel rigid needles allows for a better dose distribution with more homogeneity, as they are equidistant. Lip carcinomas are usually exophytic, and the addition of needles fixed with a template placed outside the tissue allows coverage of protruding lesions. Any oedema or reduction of tumor volume during days of treatment does not impact dosimetry, in contrast with potential dosimetric changes of the plastic tubes that have been used.
Currently, the largest study of HDR using rigid needles involved 106 patients, and results showed an impressive, preserved function in 100% of cases [9]. Ten-year tumor control was achieved in 100% in T1 tumors, 93.6% in T2 tumors, and 79% in T4 tumors. No cases of lip and bone necrosis were recorded. The same results for tumor control were achieved with LDR and plastic tubes, but with a higher rate of complications. Treatment is administered in 5 days, B.I.D. 4.5-5 Gy × 9 fractions. Elective cervical or radical neck treatment may be postponed until a week after BT, when the implant has been removed [10].
Consequently, HDR-BT is one of the main options for radical treatment of lip carcinoma [9]. In cases of close margin (< 5 mm), margin involvement, or perineural involvement, post-operative BT is recommended as adjuvant treatment.
This pictorial essay would illustrate the technique of implanting rigid needles in post-operative cases of early and advanced carcinomas of the lip.
Technique for the insertion of rigid needles in post-operative cases or early T1 tumors
Sedation and local anesthesia with lidocaine or mepivacaine of the sub-mental nerves for the lower lip and infra-orbital nerve for the upper lip are used.
The insertion technique follows a triangular configuration, using a template with perforated methacrylate plates forming equilateral triangles with a side of 10 mm (Figure 1). A single-triangle may be sufficient for small tumors (T1). The most superficial needle is first inserted along the border between the labial mucosa and the skin. The insertion and exit points should be 1 cm away from the tumor to avoid irradiation of the entry points in the skin. The second needle is then inserted into the internal commissure, where it joins the internal labial mucosa, in a parallel and equidistant manner. The third lower needle is easier to place, as it completes the triangle with the first two needles, or can even be omitted if the tumor is covered by a single-plane. An additional needle can be inserted if the surgical scar extends further, using a larger template and maintaining the triangular shape (Figures 2, 3).
Fig. 1
Distribution of needles arranged in templates with equilateral triangles. A) Three needles to treat early tumors; post-operative case with an extra needle to cover the scar. B) Advanced tumors with needles outside the tissue fixed with templates. B right) Correct placement of L-shape lead shield (from Guinot JL et al. Brachytherapy 2013; 12: 528-534 [9])
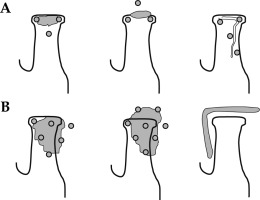
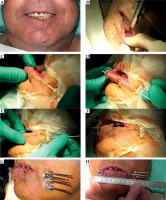
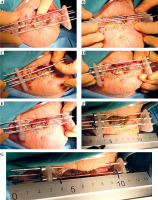
Fig. 2
Post-operative brachytherapy after wedge surgery with positive margin. A) Linear scar; B) Sub-mental nerve anesthesia; C, D) Insertion of three needles in the shape of a triangle, E, F) Insertion of three needles in the shape of a triangle, G) Additional needle is inserted to cover the lower part of the scar and fixed with screws, H) CTV is drawn on the skin, and the distance for the first and last position of the source from the tip of the needle is measured with a ruler (MicroSelectron)
Technique for the insertion of rigid needles for advanced tumors
Under sedation and analgesia, local anesthesia of the sub-mental or infra-orbital nerves is performed. Local anesthesia should also be applied in the region of commissure to thicken the tissue, and at the points of entry and exit of the needles, applying the template.
Parallel metallic needles are placed in an equilateral triangular arrangement starting from the most affected side. According to our experience, we recommend covering the commissure, even if uninvolved when treating large tumors, and to go through the inner and upper part of the lip. The exit point should be at least at 1-1.5 cm away from the tumor. The second and third needle form a triangle as in small tumors. Then, as the tumor is larger, additional needles can be added using this configuration, leaving one or more out of the tissue, held in place with the templates. More needles are inserted to ensure that the entire tumor is covered. If > 3 mm of tumor protrudes between two needles, an additional one should be placed to avoid missing clinical target volume (CTV). In these cases, a bolus or tissue equivalent material is used during the sessions to fill any air gaps, and ensuring that the dose reaches the surface of the tumor (Figures 4, 5).
Fig. 4
A) T3 squamous carcinoma of the lower lip; B) Local anesthesia is added to cover the commissure; C, D) The first needle is inserted 2 cm from the gross tumor; E, F) The second needle is inserted 1 cm apart; G) The third needle is inserted to cover the lower part of the tumor; H) More needles are added outside the tissue to cover the gross tumor
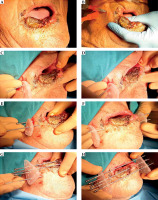
Fig. 5
A, B) We tested if more needles are needed, no more than 3 mm of tumor should protrude from the needles; C, D) The needle in the air is fixed with screws on both sides of the template; E) All the needles are fixed, but the ones in the air can be removed after the fraction and replaced in each session; F, G) CTV is drawn and the distance from the start of the needle is measured with a ruler (Flexitron)
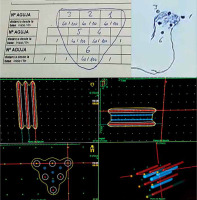
Dosimetry
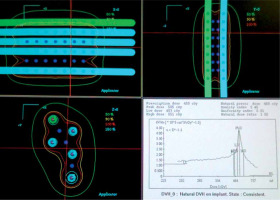
The CTV includes gross tumor volume (GTV) with a margin of 10 mm, marked on the patient’s body. The distance from the start of the needle (Flexitron) or the tip of the needle (MicroSelectron) is measured with a rigid ruler, to calculate the first and last active position. As the rigid needles are fixed with the templates, a theoretical calculation can be performed. Treatment planning is done with stepping source dosimetry system. Individualized reconstruction with orthogonal X-rays or computed tomography scan (CT) is not required. The dose is normalized to the A points as in the Paris dosimetry system, and the points of lowest dose are in the center of triangles. Geometric optimization is carried out and the prescription is at the 90% isodose, which achieves the highest homogeneity. The calculation is theoretical, and a radiation physicist completes it in a few minutes (Figure 6). A dose per fraction of 5 Gy is recommended to achieve 45 Gy in large tumors. In patients without gross macroscopic tumors, or with early tumors, the dose is reduced to 4.5 Gy in 9 fractions for a total dose of 40.5 Gy [9].
Fig. 6
We make a scheme, and dosimetry is calculated without the need for a planning CT scan, since the entire tumor is included in the volume covered by the needles. The irradiation volume does not change even though the tumor shrinks during the days of treatment due to permanent parallelism of the needles. With a larger template, the necessary needles can be added in triangles to cover any tumor size
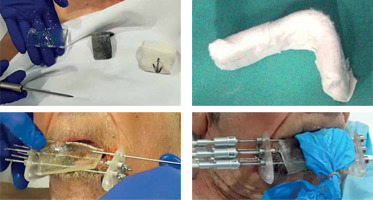
Treatment
Before each fraction, a custom-made L-shaped lead shield is placed to protect the opposite lip, tongue, and underlying mandible from radiation. Needles out of the tissue are removed and repositioned prior to the next session, to facilitate nutrition and increase patient’s comfort (Figure 7).
Fig. 7
An L-shaped lead shield is made, covered with gauze to prevent secondary electrons, and placed during each session to protect the upper lip, tongue, and teeth. In the first fraction, bolus material is placed to fill the gap. The needles outside the tissue are placed in each session, and are withdrawn at the end for the comfort of patient
Patients remain in the hospital for the full duration of HDR-BT treatment for logistical reasons without the need for special nutritional support. The nine fractions are administered twice daily, at least 6 hours apart, to deliver the entire treatment in 5 days, Monday to Friday. We did not observe bleeding or infection during the days of treatment, and antibiotic prophylaxis was not used. The needles are removed without the need for further anesthesia with gentle compression to prevent bleeding, and the patient is discharged from the hospital on the last day of treatment (Friday).
Two or three weeks after the end of treatment, in more than 80% of cases, severe mucositis may appear in the treated area. Patients should be warned about this acute effect, which resolves completely with topical treatment, such as carbenoxolone gel, in six weeks (Figure 8).
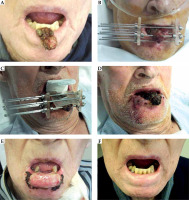
Fig. 8
A) Squamous carcinoma T2N0 (3 × 3 × 2 cm); B) Implantation of 7 needles, two of them outside the tissue; C) Bolus material and lead shield to treat 5 Gy × 9 fractions; D) Just after removing the needles, the tumor shrinks; E) Severe mucositis at three weeks; F) Excellent cosmetic and functional results 9 months after the implantation
Discussion
Interstitial brachytherapy is an invasive but highly effective treatment for lip carcinoma. This technique using rigid needles and templates is straightforward to learn, and is practical and safe with appropriate training. It is not difficult to perform good implants, because if the inserted needles do not adequately cover the tumor, additional needles can be added outside the tissue, to obtain good dosimetry.
The technique with plastic tubes is similar, but uses hollow needles with open ends, to allow replacement with the plastic tubes. Patient’s discomfort of carrying the needles is compensated by how quickly the treatment is completed, in five days. When using rigid parallel needles, it is easier and faster to calculate the dosimetry, taking only 10-15 minutes. This high homogeneity achieved makes it possible to give high doses of 5 Gy per fraction. Whereas, if plastic tubes are used, it is mandatory to perform a planning CT scan and define a CTV. In these cases, we suggest using lower doses, no more than 4 Gy per fraction to avoid complications, thus lengthening the total treatment time, with plastic tubes in place for 10 to 12 days.
Mucositis is severe, but the cosmetic and functional results are excellent. BT allows the preservation of function by avoiding the reduction of the opening of the mouth or microstomia, which surgical intervention causes when removing tissue. Recovery is complete and with fewer long-term sequelae.
The intention of this paper was to describe the rigid needle technique to treat carcinoma of the lip, as illustrated in publications with a larger number of patients, showing excellent results. It was not the purpose of this study to discuss the convenience of using other techniques, such as plastic tubes or moulds. Depending on the experience of each center and characteristics of a patient, the most appropriate option should be chosen to achieve local control with minimal complications.
Conclusions
High-dose-rate brachytherapy with rigid needles should be considered in selected patients with lip carcinoma as the first therapeutic option. Departments with HDR brachytherapy units and experienced in interstitial BT should be offering such treatment, as it greatly benefits the patients with lip cancer diagnosis. As the technique requires an expertise and manual skills, it is important that such treatment is included in the education portfolio for the next generations of interventional radiation oncologists.