Purpose
Non-melanoma localized skin cancer (NMSC), also known as keratinocyte cancer, is the most common of all cancers worldwide and its rising incidence has been well documented in systematic population-based reports [1]. In Brazil, NMSC is the most frequent neoplasm in both men and women, with a projected incidence of 211 and 190 new cases per 100,000 inhabitants, respectively [2].
There are two most frequent lines of NMSC. The first one is the basal cell carcinoma (BCC), derived from basaloid cells and with different variant types that have greater or lesser aggressive behavior. The second – the squamous cell carcinoma (SCC) is the most frequent line of NMSC [3].
Treatment options for NMSC include surgical interventions, radiotherapy, and cryotherapy, among other. In general, the choice of treatment modality is driven by the risk assessment of lesions. The use of high-dose-rate (HDR) brachytherapy in the treatment of NMSC it is not new. Köhler-Brock et al. published in 1999 their 10-year experience and reported 91% of complete remission for a wide range of skin tumors, with a total dose between 30 and 40 Gy [4]. Several other studies using HDR to treat NMSC reported control rates ranging from 92.5% to 100% [5,6,7,8].
In the present study, we describe our single institutional experience of treating NMSC with HDR using Leipzig-style applicators.
Material and methods
This is a retrospective review of all patients treated with radical intent and HDR brachytherapy for BCC and SCC, using Leipzig-style applicators (Varian Medical Systems, Palo Alto, CA, USA) at the Radiation Oncology Department, AC Camargo Cancer Center, from March 2013 to December 2018. Patients with localized non-metastatic BCC or SCC, confirmed histological study, had refused or were unsuitable for surgery were considered for inclusion. The approval for this study was obtained from the Institutional Research Ethics Board. A total of 71 patients, with 101 lesions and median age 80 (range, 51-102) years old were treated during the period and included in the analysis.
The patients were immobilized with plastic masks or fixation devices whenever necessary and only after that, the applicators were positioned.
The area to be treated was defined as the visible lesion plus a safety margin of 10 to 15 mm around the lesion, depending on the tumor size, location, and histological type. Treatments prescribed (100% prescribed dose) at 3- or 5-mm in depth (median doses at 0.125 mm) from skin surface for each prescription were 120% and 150%, respectively. Tumors equal to or less than 20 mm in diameter had the prescribed dose at 3 mm in depth, while in lesions with diameters greater than 20 mm, the prescribed dose was applied at 5 mm in depth.
To compare different treatment schedules, the biological effective dose (BED) and equivalent dose to 2 Gy (EQD2) were calculated using linear quadratic model, with α/β of 10 for the skin tumors.
Treatment endpoints included treatment efficacy in terms of tumor local control (LC), with complete response (CR), skin healing or stable disease (SD), a decrease of 30% or higher from baseline, and acute and late skin reactions. The characteristics of lesions are shown in Table 1.
Table 1
Characteristics of the tumors
| Variable | n | % | Range | Median |
|---|---|---|---|---|
| Tumor size (mm) | 5-42 | 32 | ||
| ≤20 | 26 | 25.7 | ||
| > 20 | 75 | 74.3 | ||
| Anatomical site | ||||
| Face | 40 | 30.8 | ||
| Extremities | 7 | 6.9 | ||
| Trunk | 14 | 13.9 | ||
| Scalp | 19 | 18.8 | ||
| Other | 21 | 20.8 | ||
| Histology | ||||
| SCC | 31 | 30.7 | ||
| BCC | 70 | 69.3 | ||
| Total | 101 | 100 |
During treatment period, the patients were evaluated weekly and after completion of treatment, the patients were followed-up with monthly intervals for the first 3 months, then at 3 to 4 months interval, up to the second year. Then, they were seen every 6 months, till the end of fifth year. Subsequently, the patients were informed about annual follow-up for at least 5 years.
Acute and late skin toxicities were evaluated weekly during and after the end of irradiation course using the Radiation Therapy Oncology Group (RTOG) criteria [9].
Statistical analysis
Survival analysis was performed using Kaplan-Meier, and Breslow statistic test was used to compare differences in LC. P-value of less than 0.05 was considered statistically significant. SPSS v.20 for Windows was applied for statistical calculations. To investigate the interaction of several variables, Cox regression model was used.
Results
The majority of lesions were classified as BCCs (69.3% or n = 70). Additional demographic details of the patients are presented in Table 2. The most frequent fractionation scheme used was 40 Gy, given in 10 daily fractions, 5 times per week (BED = 56 and EQD2 = 46.7 Gy), n = 28 (27.7%). Other fractionation schemas used are listed in Table 3. The median BED given for all patients was 59.6 (range, 39.2-68.7) and the median EQD2 was 50 Gy (range, 32.7-57.3).
Table 2
Baseline patients’ characteristics
| Variable | n | % | Range | Median |
|---|---|---|---|---|
| Age (years) | 51-102 | 80 | ||
| < 65 | 9 | 12.7 | ||
| ≥65 | 62 | 87.3 | ||
| Gender | ||||
| Male | 31 | 43.7 | ||
| Female | 40 | 56.3 | ||
| Co-morbidities | ||||
| Yes | 58 | 81.7 | ||
| No | 13 | 18.3 | ||
| Total | 71 | 100 |
Table 3
Treatment schemas
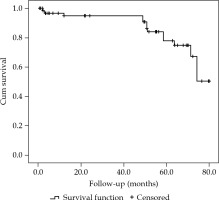
After median follow-up of 42.8 (range, 12-82) months, the 3-year overall survival (OS) for the entire cohort was 94.5% (Figure 1). A total of 7 patients had died at the time of analysis. Only three had died due to progression of their skin malignancies (all SCCs) and four died of unrelated co-morbidities.
Complete response was observed in 67 (66.3%) lesions, SD in 8 (7.9%), and no response was observed in 19 (18.8%) lesions. On follow-up, local recurrences (LR) were noted in 7 (6.9%) patients in a median time of 9 (range, 3-25) months. Of these, 3 patients had SCC of the nose, 2 of the scalp, one BCC of the forearm, and another of the ear. Three patients (2.9%) died of local or distant disease progression.
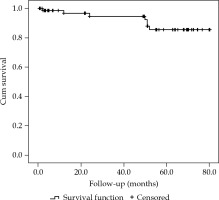
The 3-year and 5-year actuarial local control (LC) rates were 97.9% and 87.2%, respectively (Figure 2).
On univariate analysis, treatments with EQD2 less than 50 Gy (p < 0.001) and dose per fraction smaller than 3 Gy (p < 0.001) were found to be statistically significant predictive factors of a worse outcome. There was a marginally significant difference, when histological types were compared (p = 0.093), favoring the LC for BCCs. The results are presented in Table 4. On multivariate analysis, SCC had a worse prognosis over BCC (p = 0.007, HR = 2.3, CI: 1.2-6.6).
Table 4
Univariate analysis
All patients developed some degree of acute side effects graded 1 to 2. Grade 3 acute side effects were observed in 9 (8.9%) patients. Severe late side effects (grade 3), hypopigmentation, and telangiectasia were observed in 4 (3.9%) patients. No grade 4 acute or late side effects were noted in this cohort.
Discussion
The management of patients with NMSC unsuitable for or who refuse surgery, is still a matter of active investigation. The best treatment option should seek cosmetic and functional outcome. Location and size of the primary lesion are considered gold marks to define the best treatment option. The guidelines from the NCCN-2019, divide NMSC into two groups: low- and high-risk lesions. Lesions greater than 2 cm and located outside the trunk and extremities are considered high-risk. For the head and neck lesions, the cutoff size is 1 cm. Other prognostic factors are borders’ definition, first presentation in contrast to recurrent lesions, immunocompetence of individuals, previous history of radiation at the lesion site, and presence of perineural or perivascular invasion [10].
The main types of radiation used to treat NMSC are low-energy X-rays and electron beam therapy. Both have a shallow energy deposition, which is ideal for the purpose of treating the skin when compared to high-energy photons; however, both type of radiation are not widely available in Brazil. Otherwise, HDR equipment is frequent in many centers due the relative high incidence of cervical cancer in our country [11].
Low-energy photons and electrons have been used for decades to treat NMSC. Long-term control rates, including superficial X-rays (45-100 kV), orthovoltage X-rays (100-250 kV), megavoltage photons, and electron beam radiation, ranging from 87% to 100% after a follow-up of 2 to 5 years, and moderate dose fractions application are suggested [12,13,14,15,16,17].
The cup-shaped Leipzig-style applicators are available with different inner diameters of 30, 35, 40, and 45 mm, with the iridium-192 (192Ir) source having either a parallel or perpendicular orientation. This different source positioning related to the applicator allows the use of anisotropic properties of the source in calculations. We opted for applicators with parallel source positioning, not taking in account the anisotropic factors in dose calculations. The most frequent planning system used for HDR dose calculation are based on the American Association of Physics in Medicine (AAPM) TG-43, in which the scatter defect is not considered as a dosimetric parameter. An analysis of eventual differences at the prescription depth between TG-43 and Monte Carlo calculations was negligible for 192Ir, therefore no bolus over the skin was necessary [18]. As per the manufacturer’s manual, the recommended depths of prescription were 5 or 3 mm, at 0.125 mm from skin surface, with prescribed doses of 150% and 120%, respectively.
It is important to note that the dosimetry of electrons’ field over small irregular surfaces have a vast variation in the percentage of depth dose and output parameters, which also depends on the field size and electron energy used. Souransu et al. reported much better conformity with HDR when compared to electrons in these situations [19]. Furthermore, the use of HDR has some advantages over other techniques: Leipzig-style applicators are easily placed, personal radioprotection of patient is maintained, with no isolation required, and HDR can be performed on an outpatient basis. Moreover, the sessions are fast, and anesthesia is not required at most of the times.
Another paper by Sabbas et al. compared the dosimetry of conventional orthovoltage, photons, and electrons to HDR dose distribution on the skin, and observed that the last one has a sharp gradient, similar to the conventional low-energy X-ray, in contrast to the electron dose distribution. This sharp gradient allows for an application of higher dose to first millimeters of skin and lower dose to deeper tissues, which is especially helpful when treating skin cancer [20].
The recommendations for radical radiotherapy include contraindications or surgery refusal, frail patients that may not tolerate the surgical procedure, and tumor location in areas where the cosmesis and/or function may be impaired. The treatment of anatomical sites, such as the eyelids, nose, and lips have better final cosmetic and functional results with radiation when compared to local excision [21]. Among various prognostic factors suggesting a benefit of adjuvant radiation to improve LC, the status of surgical margin, tumor size, and depth of invasion are the most used ones [22].
The literature has various studies reporting the use of HDR to treat skin lesions, suggesting high LC, but no prospective randomized trial has compared the different types of radiation. Köhler-Brock et al. published their 10-year experience of 520 lesions treated with HDR using Leipzig applicators to a total dose ranging from 30 to 40 Gy and reported 91% of complete remission [4]. Gauden et al. described the results of 236 lesions treated with HDR. With a median follow-up of 66 months, they observed 98% of LC [6]. Maroñas et al. evaluated the results of 51 lesions with a mean size of 15 mm and maximum thickness of 3 mm, treated with 48-57 Gy given in 3 to 4 Gy per fraction three times a week, but using special moulds. Only five (9.8%) tumors relapsed, which was very similar to our results. Their 5-year actuarial LC rate was 89% [8]. A report from Pellizzon et al. presented the results of 13 patients treated with HDR and Leipzig applicators, showing crude and actuarial 3-year LC rates of 100% and 80%, respectively. Two of the 13 patients presented dyspigmentation in the irradiated area [11].
Given the increasing number of patients’ aging and increasing incidence of NMSC, tailored assessments for this population may be beneficial. In a study of 2,702 patients older than 65 years and treated for NMSC, the rates of treatment were no different among patients with limited life expectancy, compared with those with normal life expectancy [23].
Different hypofractionated regimens with very good local control have been reported in literature (Table 5). To date, various empirical models were created to compare the probability of gain in therapeutic ratio. The most recent ones are the BED and EQD2 calculations, but the simplified use of both do not consider the frequency of radiation during the week. In our study, we observed a trend towards better results in terms of LC when using doses per fraction of more than 3 Gy. Schedules found in literature are similar to electron beam, ranging between 3 and 5 Gy per fraction, 2-3 days per week in 4-5 weeks [4,5,6,8,11,24,25,26,27]. What is more, the schedule used in our study has a relative longer median follow-up time when compared to other published series. Using the Kaplan-Meier method, we estimated 3- and 5-year LC rates of 97.9% and 87.5% for the entire cohort.
Table 5
Recent published results using HDR to treat NMSC
| Author (reference) | No. of lesions | Follow-up (months) | Total dose/ fraction (Gy) | LC (%) | Acute side effects* | Late side effects* |
|---|---|---|---|---|---|---|
| Köhler-Brock [4] | 510 | 120 | NA | 91 | NA | NA |
| Guix [5] | 117 | 60 | 60-66/1.8 | 87 | G4 10% | G3 8% |
| Gauden [6] | 236 | 66 | 36/3 | 98 | G1 71% G2 34% | G3 5.5% |
| Maronas [8] | 51 | 45 | 48-57/4-3 | 89 | NA | NA |
| Pellizzon [11] | 13 | 36 | 20-60/3-6 | 80 | G1-2 30.8% | G2 23% |
| Delishaj [24] | 57 | 12 | 40-50/8-5 | 96.2 | G1-2 63.2% | G2 19.3% |
| Arenas [25] | 102 | 33 | 45-57/3 | 94.9 | G1-2 57.3% | G4 2.2% |
| Allan [26] | 13 | 18 | 45/5.6 | 100 | NA | NA |
| Montero [27] | 11 | 15 | 44-48/4 | 100 | NA | NA |
* Side effects graded according to RTOG score [10]
As reported in literature, HDR brachytherapy treatment is very well tolerated with excellent cosmetic results despite using applicators. Moreover, excellent cosmetic outcomes and acceptable acute and late side events were observed even in elderly patients using hypofractionated regimen of 40-50 Gy, delivered in 8-10 fractions, 2-3 time weekly [4,8,11,24,25,26,27]. Even though cosmetic results are very important, they were not evaluated in this study, because grading is subjective and reporting systems across studies are very heterogeneous. Furthermore, the population of our study was relatively old, and their perceiving of cosmetic outcome may differ substantially from younger patients.
The most common early side effects due to HDR treatment are erythema and edema, both expected to occur in some degree in all patients. Other less frequent acute complications are rash dermatitis, pruritus, desquamation, and in rare cases, ulceration. In our cohort, we observed an incidence of 12.7% and 5.6% of acute and late side effects, respectively. Late side effects were mainly hypopigmentation and telangiectasia. No grade 4 acute or late complication was observed in our cohort. As per definition, late side effects appear 6 months after HDR treatments and often consist of atrophy, pigmentation change, hair loss, telangiectasia, fibrosis, and less frequently, chronic ulceration. Gauden et al. [6] reported that late skin hypopigmentation changes were observed in 13 cases (5.5%). In our series, we observed only less than 2% of hypopigmentation (n = 2) and telangiectasia (n = 1).
Prospective RCTs comparing various surgical modalities or with RT modalities are limited, despite the fact that radiation has been used with great success for many decades. One randomized trial comparing different treatment modalities, published in the 90’s, suggested that radiotherapy was inferior to surgery in terms of local control and cosmesis [28]. Conversely, a recent meta-analysis of 58 studies with around 21,000 patients concluded that local control was similar among surgical and radiation modalities at one year [29].
Conclusions
High-dose-rate brachytherapy offers a convenient treatment schedule for patients and is associated with excellent LC. The most effective regimen, in terms of dose and fractionation, to treat superficial NMSC with HDR remains uncertain, but a moderate minimum EQD2 of 50 Gy should be used, given with 3 Gy fractions or higher.