Purpose
Radical radiotherapy treatment for pelvic recurrence of uterine cervical cancer poses a clinical challenge due to proximity of organs at risk (OARs) to the tumor site. In particular, pelvic sidewall recurrences carry significant morbidity risk, prohibiting aggressive treatment due to involvement of large vessels and nerves. In such cases, an implementation of curative surgery is also limited [1], and treatment outcomes of external beam radiotherapy (EBRT) with or without chemotherapy, are similarly unsatisfactory [2]. Interstitial brachytherapy (ISBT) may provide an effective alternative for this poor-prognosis population. ISBT combines a lesion-specific approach of surgery with a tumoricidal capability of radical radiotherapy by escalating biologically effective dose to the treatment target, while ameliorating conformity. Versatility of intra-target dose modulation inherent to brachytherapy offers greater control and ability to directly deliver higher doses to the total tumor, while selectively reducing the dose to OARs. Furthermore, technical advances, such as image-guided implantation, template-based approaches, and anatomy-oriented treatment planning have shown significant improvements in the treatment of recurrent uterine cervical cancer with low-dose-rate (LDR), pulsed-dose-rate (PDR), and high-dose-rate (HDR)-ISBT [3,4,5,6,7,8,9,10,11,12,13,14,15]. Our own experience with three-dimensional (3D) image-guided HDR-ISBT for recurrent uterine cervical and endometrial cancers after primary surgery is reflected in the published results of 56 patients with 3-year local control of 75% [16,17].
In spite of the enormous potential of HDR-ISBT, various factors, including complex lesion shapes and pubic arch interference may impair implantation and adequate irradiation, especially in pelvic sidewall recurrences. In order to improve the outcomes of such patients, we implemented a new hypoxic radiosensitizer in the radiotherapy of unresectable carcinomas (Kochi oxydol-radiation therapy for unresectable carcinomas – KORTUC). Ogawa et al. demonstrated that radioresistance of the human osteosarcoma cell line HS-Os-1 arises from low levels of reactive oxygen species (ROS) formation following irradiation, which in turn may result from a strong free radical scavenging ability of the cells, particularly scavenging of hydroxyl radicals. Therefore, they examined the effects of various doses of irradiation in the presence of 0.1 mM hydrogen peroxide in a culture medium. They found that irradiation with 10 or 20 Gy, in the presence of 0.1 mM hydrogen peroxide, induced ROS formation, oxidative DNA damage, dysfunction of the mitochondrial membrane potential, and early apoptotic changes in the human osteosarcoma cell line HS-Os-1. Interestingly, ROS formation and oxidative DNA damage were scarcely seen in a response to irradiation of up to 30 Gy, as shown in their previous study. The authors concluded that the modality of irradiation combined with a low concentration of hydrogen peroxide (0.1 mM) may be found useful in clinical radiotherapy [18]. Based on these results, they clinically evaluated the effectiveness of this enzyme-targeting radiosensitization treatment, using a superficial coating of hydrogen peroxide (the KORTUC I method) and intra-tumoral injections of sodium hyaluronate mixed with hydrogen peroxide (the KORTUC II method) [19,20]. However, in comparison to EBRT, there are limited data on a hypoxic radiosensitization approach with ISBT. In this report, we describe a case of pelvic sidewall recurrence treated with HDR-ISBT combined with KORTUC II.
Clinical case
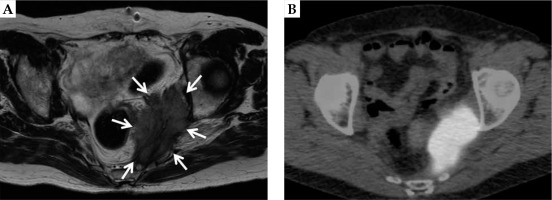
A 63-year-old female patient was referred to our department with a pelvic sidewall recurrence of uterine cervical cancer. Initially, the patient was treated with two cycles of neoadjuvant chemotherapy (intra-arterial cisplatin infusion and intravenous irinotecan). Irinotecan 70 mg/m2 on day 1 and 8, and cisplatin 75 mg/m2 on day 2 were administered as one cycle. Subsequently, she underwent a radical hysterectomy (ypT2a2 ypN1 cM0 pR0, squamous cell carcinoma), followed by six cycles of adjuvant chemotherapy consisting of paclitaxel plus carboplatin. Paclitaxel at 175 mg/m2 and carboplatin at an area under the curve of 5 were administered as one cycle. Twenty-three months after the surgery, the patient developed an unresectable, left-sided pelvic sidewall recurrence. Her clinical symptomatology consisted of perianal pain. Magnetic resonance imaging (MRI) revealed a large lesion of 55 × 25 × 80 mm (Figure 1A), with an associated pathologic uptake of 18F-2-deoxy-glucose (FDG) in positron emission tomography (PET)/computed tomography (CT), without distant metastases (Figure 1B). Tumor marker (SCC) was elevated to 6.7 (normal range, < 1.5). Even though a biopsy was not performed, the lesion was clinically diagnosed as a recurrence.
Fig. 1
A) Magnetic resonance imaging depicting the left-sided uterine cervical cancer recurrence. In the T2-weighted sequence, the large tumor (55 × 25 × 80 mm) at the left pelvic sidewall is clearly demarcated (white arrows). B) Positron emission tomography/ computed tomography scan image of the same patient. A pathologic uptake of 18F-2-deoxy-glucose (FDG) is shown in correspondence to the MRI findings
After interdisciplinary assessment, followed by informed consent, the patient was offered EBRT to the whole pelvis up to 50 Gy in 25 fractions, with weekly intravenous cisplatin (40 mg/m2) injections. We injected KORTUC II in total three times, with two times performed in EBRT and final injection during ISBT. KORTUC II was additionally injected into the tumor lesion at day 21 (dose point, 42 Gy) and day 24 (dose point, 48 Gy) during the EBRT. In our hospital, 0.5 ml of 3% hydrogen peroxide, 2.5 ml of sodium hyaluronate (Adant Dispo intra-articular injection®), and 1 ml of 1% lidocaine (Xylocaine®) were mixed in a single syringe to formulate KORTUC II, and 1 to 3 syringes (4-12 ml) were injected depending on the tumor size. If the tumor diameter was less than 30 mm, only one syringe (4 ml) of KORTUC II was injected at each time point. If the tumor diameter was 30-60 mm, 2 syringes (8 ml) of KORTUC II were injected at each time point, and if the tumor diameter was more than 60 mm, 3 syringes (12 ml) of KORTUC II were injected at each time point. This method was similar to that used in a previous phase I clinical trial started in 2017 by Nimalasena et al. [21]. Freehand transvaginal intratumor injection was performed under local anesthesia. We inserted an injection needle with the right hand and guided it to the tumor using left hand from inside the rectum.
ISBT was performed as a boost under general anesthesia, 18 days after the completion of EBRT. Our HDR-ISBT technique has been described in detail elsewhere [22]. In short, 13 flexible needle applicators (ProGuide sharp needle®; Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden) were implanted transperineally using a freehand technique without template guidance under transrectal ultrasonography (TRUS). Subsequently, the applicators were cut short in order to allow the patient to sit and/or stand up. The KORTUC II application was then performed (Figure 2A), followed by CT imaging for anatomy-oriented HDR treatment planning (slice thickness 2 mm, AquilionLB®, Canon Medical Systems, Tochigi, Japan). Since catheter distribution was not deemed optimal, an additional applicator was implanted under CT guidance. KORTUC II was injected between applicators because the area between applicators showed a relatively low-dose irradiated area, which was similar to the basal dose points.
Fig. 2
A) Computed tomography of the same patient with 14 flexible needle applicators in situ. The white arrow indicates oxygen gas through the injection of sodium hyaluronate mixed with hydrogen peroxide into the tumor (the KORTUC II treatment). B) Isodose distribution of the interstitial brachytherapy. The 100%-isodose line indicates the prescribed dose (5 Gy, black dotted line) covering the CTV (black solid line) without delivering excessive doses to the rectum (R) and the sigmoid colon (S). Povidone iodine gel was inserted into the rectum in order to visualize the mucosal surface clearly
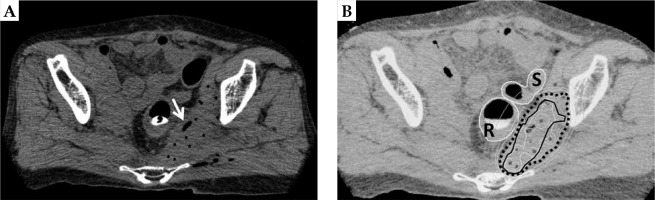
The final treatment plan was carried out using CT/MRI co-registration for improved target and OARs detection [23] (Figure 2B). Povidone iodine gel was injected into the rectum to assist in visualization of the mucosal surface by a CT component. The treatment plan included measurement of gross tumor volume and OARs delineation with clinical target volume (CTV). Planning target volume (PTV) was generated from a CTV by adding a 10 mm cranial margin [24,25].
Treatment plan evaluation was based on dose-volume histogram (DVH) analysis under consideration of the dose covering 90% [D90 (CTV)], 98% [D98 (CTV)], and 100% of the CTV [D100 (CTV)]. Our dosimetric goal was D100 (CTV) ≥ planning aim dose (PAD), while allowing for D100 (CTV) < PAD in case of excessive OARs doses, in which our dosimetric goal was D90 (CTV) or D98 (CTV) > PAD. For DVH-based OARs evaluation, we considered the minimum dose received by the maximally irradiated 2 cc (D2cc). The HDR-ISBT protocol consisted of 5 Gy fractional doses b.i.d. up to a total physical PAD of 25 Gy over 3 days, with an interfraction interval > 6 h. After the final HDR treatment, the applicators were explanted. Considering an α/β = 10 Gy, the prescribed PAD generated a biological effective dose of 97.5 Gy, with 10 Gy making an equivalent total dose in 2 Gy fractions (EQD2) of 81.3 Gy. In accordance with the DVH analysis, the volume to be irradiated with the prescribed dose as well as the volume covered by the 150% isodose amounted to 204.6 cc and 87.7 cc, respectively. The CTV and PTV volumes were 89.7 cc and 118.3 cc, respectively. The D90 (CTV), D98 (CTV), and D100 (CTV) were 6.0, 5.0, and 3.5 Gy per fraction, respectively. Similarly, D90 (PTV), D98 (PTV), and D100 (PTV) were 5.1, 3.8, and 2.8 Gy per fraction, respectively.
Dose limits for total EQD2 of OARs were < 90 Gy for the bladder, < 75 Gy for the rectum, < 75 Gy for the sigmoid colon, and < 75 Gy for the small bowel (Table 1). This criterion was based on the EMBRACE II study [26]. Dose constraints of the sciatic nerve was not determined.
Table 1
The dose limits of organs at risk for treatment planning. EQD2 is calculated using α/β = 3. The total EQD2 include 50 Gy/25 fractions delivered by EBRT
| Bladder D2cc | Rectum D2cc | Sigmoid colon D2cc | Small bowel D2cc | |
|---|---|---|---|---|
| Dose limits | < 90 Gy | < 75 Gy | < 75 Gy | < 75 Gy |
The D2cc values were 2.1, 4.1, 3.2, and 2.0 Gy per fraction for the bladder, rectum, sigmoid colon, and small bowel, respectively. With the addition of EBRT, the total EQD2 of D2cc values were 60.7, 79.1, 69.8, and 60 Gy per fraction for the bladder, rectum, sigmoid colon, and small bowel, respectively.
The D0.1cc value for the left sciatic nerve was at least 4.1 Gy. The total EQD2 of D0.1cc value for the left sciatic nerve was at least 79.1 Gy. However, the left sciatic nerve could not be completely isolated because it was surrounded by the tumor lesion. Hence, the D0.1cc values may be higher than the above-mentioned values.
All treatments were performed using an Iridium-192 (192Ir) HDR-afterloading system (MicroSelectron-HDR; Elekta AB, Stockholm, Sweden), with an apparent initial source activity of approximately 370 GBq.
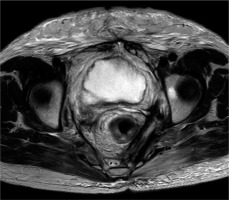
The patient was followed up regularly and demonstrated very good clinical response. The follow-up MRI at 8 months after completion of treatment showed a complete radiological response according to RECIST criteria (Figure 3). The last follow-up CT at 32 months after ISBT, demonstrated enduring local control (LC) without newly diagnosed intrapelvic deposits. SCC tumor marker decreased to 0.5.
Fig. 3
Magnetic resonance imaging taken 8 months after interstitial brachytherapy as a part of follow-up. In the T2-weighted sequence, a long-term complete clinical remission can be verified
Regarding toxicity, adverse events were scored according to the terminology criteria for adverse events version 4.0 (CTCAE v4), and no acute complications ≥ grade 3 were observed after HDR-ISBT combined with KORTUC II. In terms of late complications, the patient described left-sided sciatic neuralgia at 7 months after the treatment, which was scored as grade 2, and still persisted at the time of writing. Synchronous grade 2 left lower limb lymphedema and urinary incontinence developed, showing no improvement during further follow-up. Thigh circumference was measured at the level of 10 cm below the femoral head, and the difference increased from 1.5 cm to 3.3 cm from pre-radiotherapy to 29 months after ISBT. Urinary incontinence was influenced by pyelonephritis; however, slight stress incontinence was also present at the time of writing this report. Repeated grade 3 pyelonephritis occurred at 11-, 24-, and 26-months post-treatment, and fully resolved after temporary intravenous antibiotic treatment.
Discussion
The management of recurrent uterine cervical cancer after definitive previous treatment, poses a challenge to the clinician. In fact, locoregional disease progression represents the most common cause of death in this gynecologic malignancy [27], with therapeutic options being limited and rarity of high-quality evidence. However, it should be emphasized that if locoregional disease is left untreated, the prognosis and quality of life are quite poor.
Although surgical resection constitutes the primary curative option when recurrent disease is resectable, but in reality, only a small group of patients is suited for this approach [28]. Few reports exist concerning the resection of pelvic sidewall recurrent cancer [29,30]. Höckel reported on laterally extended endopelvic resection for pelvic wall recurrences [29]. He surgically treated 18 patients with or without post-operative radiotherapy, and a 3-year survival rate was 40%. However, this patients were carefully selected prior to surgery according to indications that patients presented with infra-iliac lesions, the tumor was ≤ 5 cm, and had developed > 5 months after a primary treatment.
Chemotherapy alone, with or without anti-vascular endothelial growth factor monoclonal antibody, showed a median survival of 13-17 months [31].
When irradiation is the only remaining salvages option, ISBT has demonstrated effectiveness in the management of recurrent uterine cervical cancer [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. However, there are no well-defined recommendations for selecting patients for interventional radio-oncologic treatment, and each case is analyzed individually. For example, Jensen et al. treated 34 patients with locally advanced or recurrent gynecologic cancer using PDR-ISBT, and a 2-year survival rate was 63%. PDR-ISBT combines the advantages of both LDR-ISBT (relatively lower dose-rate, which is theoretically safer than HDR-ISBT) and HDR-ISBT (no radiation exposure for medical staff) [15]. However, in Japan, the physician is obliged to accompany the patient during all irradiation sessions, which makes this procedure too difficult to accomplish and, as a result, PDR-ISBT is not used in Japan.
Some prognostic factors have been described for salvage radiotherapy with tumor size playing an important role [6,8,11,13,14]. Charra et al. examined the use of LDR-ISBT in a treatment of vaginal recurrences of uterine cervical cancer, and showed that tumor diameter was a significant prognostic factor for a 5-year overall survival, which decreased from 63% to 37% if a recurrent lesion measured ≥ 4 cm [6]. Gupta et al. reported similar results on HDR-ISBT for locally advanced or recurrent gynecological malignancies, stressing tumor size as a significant prognostic factor with deterioration of a 3-year LC from 89% to 0% when a recurrent lesion volume was ≥ 100 cc [8].
In addition to lesion size, tumor location seems also to be associated with clinical outcome [3,4,7,11]. Tan et al. reported on 100 patients treated for post-operative recurrent uterine cervical cancer using radical radiotherapy with or without chemotherapy, and found a 5-year survival rate of 42% for centrally located lesions vs. 15% for peripheral recurrences [4]. Likewise, Ijaz et al. reported on radiotherapy of pelvic recurrences after radical hysterectomy for cervical carcinoma. Their study encompassed 43 patients treated with or without chemotherapy, yielding a 5-year survival rate of 69% for central recurrence alone vs. 18% for recurrent tumors with sidewall extension [7].
In our case, the volumetrically calculated tumor volume was 89.7 cc with deep extension into the pelvic sidewall. From this perspective, we considered KORTUC II a meaningful treatment option to restrict dose prescription through enhanced radiosensitivity [19,20]. Although our dose fraction schedule was slightly higher than those of other institutes (EQD2 of 75.5-79.6) [32], the biological effects compared favorably with the recommended schedules in the ABS guidelines. Ogawa et al. reported the use of KORTUC II for unresectable or recurrent neoplasms, including sarcoma and malignant melanoma [20]. They treated 52 patients with a total of 53 lesions by EBRT. Their cohort included 31 patients with breast cancer and 8 with soft tissue sarcoma. The EBRT dose regimen was 49.5 Gy in 18 fractions for breast cancer and 54 Gy in 27 fractions for soft tissue sarcoma. The mixed complete response rate was 57% with a 2-year disease-free survival of 37%, although the vast majority of cases consisted of large tumors and radioresistant histologic subtypes.
However, in cases with deep-seated large tumor like our patient, it is relatively difficult to inject KORTUC II homogeneously into the whole tumor area and, in some patients, the treatment may spread throughout the body causing damage to other organs. Additional care is required to ensure the safety of KORTUC II injections when treating patients with deep-seated large tumors, which require the use of a large number of long needles, as both the KORTUC II injection and needle applicator implantation may cause mechanical trauma, such as small bowel perforation [33]. The freehand injection technique used to administer KORTUC II during EBRT period must always be carried out in conjunction with CT/TRUS guidance as a standard procedure. We injected KORTUC II and implanted needle applicators with CT and TRUS guidance at the time of ISBT, as this constitutes a safer and more reproducible technique. Such image-guided implantation is also important in order to enable dose escalation to tumor while reducing dose to OARs. Furthermore, KORTUC II injection in a relatively lower dose irradiated areas, such as between applicators, offers a more effective method. We believe that further studies on the application of KORTUC II, focusing on overcoming difficulties associated with delivering the prescribed radiation doses to large deep-seated tumors without any cold spots, are necessary. Nimalasena et al. recently completed a phase I trial using EBRT with KORTUC II in locally advanced breast cancer patients [21]. Although, all tumors were ≥ 30 mm, and 11 of the 12 patients maintained a partial or complete response (median follow-up, 12 months). Even though the KORTUC II procedure is easy for the treatment of superficial lesions, a safer and more stable technique remains to be found for large deep-seated tumors worldwide.
Based on the proven performance of KORTUC combined with radiotherapy, we offered KORTUC II treatment during EBRT and ISBT to our patient. After obtaining an informed consent, we carried out the procedure, which yielded improved radiosensitivity of hypoxic tumor cells that typically occurs during whole-pelvis EBRT, and ameliorated the radiation response in potential ‘cold spot areas’ caused by HDR-ISBT. The driving force behind this strategy was the provision of a curative treatment in a case of local pelvic recurrence of uterine cervical cancer with negative prognostic factors. Currently, 32 months post-treatment, the patient remains free of disease progression, with no further late severe adverse events. The toxicity profile is in line with the literature, in which gastrointestinal and genitourinary adverse events ≥ grade 3 are reported in up to 29% after radiotherapy for gynecological intrapelvic recurrences, respectively [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17].



