Purpose
Locally advanced cervical cancer (LACC) remains a prevalent global disease, for which brachytherapy (BT) is of utmost importance for primary tumor control and overall survival [1]. The evolution of brachytherapy over the past two decades, driven by advances with image-guided brachytherapy (IGBT), for cervical cancer has resulted in excellent local control, survival, and toxicity outcomes [2]. Following the implementation of IGBT, there has been growing interest in the development of hybrid BT applicators that allow increased dose coverage of the parametria and pelvic sidewall for tumors not adequately treated with an intracavitary device [3]. Although hybrid intracavitary/interstitial (IC/IS) applicators are now in clinical use, there is limited large scale data outside of the EMBRACE studies surrounding clinical outcomes, as they relate to tumor and organs at risk (OARs) dose and tumor volume metrics. Specifically, there is a lack of data in patients treated with a tandem and ovoids hybrid brachytherapy approach. We sought to demonstrate the contemporary clinical outcomes of LACC patients treated with IC/IS BT using a hybrid tandem and ovoids device, and their association with tumor and OARs doses, and tumor volume metrics at brachytherapy.
Material and methods
This retrospective analysis was performed following approval from our institutional review board. An institutional database was queried for LACC patients treated with concurrent external beam radiation therapy (EBRT) and chemotherapy followed by hybrid IC/IS BT from 2010-2017. EBRT was performed using computed tomography (CT)-based image-guided IMRT, with pelvic organ motion managed with an internal target volume (ITV)-based approach. Patients were simulated with a full and empty bladder to account for motion of the vagina, cervix, and uterus. Gross tumor volume was delineated using CT, positron emission tomography (PET/CT), and magnetic resonance imaging (MRI) fusion as per the preferred institutional standard. A clinical target volume (CTV)-cervix included a 0.5 cm expansion on the gross tumor volume. This was done on both full and empty bladder CT scans separately. A CTV including the CTV-cervix plus the vagina and uterus was generated on full and empty bladder scans separately, then combined into a single ITV-VCU (vagina cervix uterus). A 1.0 cm expansion was then applied to generate the PTV-VCU. A separate CTV-LN (lymph node) of the pelvic and para-aortic (based on physician discretion) lymph nodes, and parametria was contoured with a 0.7 cm expansion to PTV-LN. Our institutional practice is to include para-aortic lymph nodes if there is an evidence of involved pelvic or para-aortic lymph nodes on imaging. Any gross lymphadenopathy had a 0.5 cm expansion to a CTV, which was included in the CTV-LN. The PTV-VCU and PTV-LN were combined for a total PTV, which was prescribed a dose of 45 Gy in 1.8 Gy fractions. A simultaneous integrated boost was given to the primary tumor and any gross lymphadenopathy to a dose of 50 Gy in 2 Gy fractions. Following the simultaneous integrated boost to 50 Gy, any gross lymph nodes alone were sequentially boosted to a dose of 54-60 Gy, as they were not treated in the brachytherapy boost. The sequential lymph node boost dose was prescribed at the discretion of treating radiation oncologist on an individual basis.
Brachytherapy
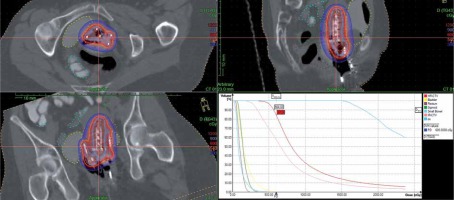
Brachytherapy was administered in 6-8 Gy fractions over 3-5 insertions via a hybrid tandem and ovoids applicator utilizing an 192Ir afterloader high-dose-rate system. The Utrecht applicator (Elekta) was used for all cases in this series (Figure 1). Cervical Smit sleeve insertion was performed in the operating room prior to BT fractions, generally in the last weeks of EBRT. BT insertions were performed in the outpatient setting, with the use of a narcotic and anxiolytic regimen, and viscous lidocaine for pain control or conscious sedation. Tandem and ovoids were inserted followed by placement of a vaginal balloon posterior to the ovoids in the vaginal canal. In general, the tandem is inserted through the vaginal canal to pass through the cervical Smit sleeve, followed by placement of the ovoids with interstitial guide tubes in place. CT scan confirmation of the placement was used in all insertions, as well as, calculation of distance of interstitial needle insertion and adjustments. Placement of interstitial needles was decided upon for improved dosimetry based on parametrial or sidewall disease, or bulky disease at the time of brachytherapy. CT image-based contouring, treatment planning, and plan evaluation were performed according to the GEC ESTRO guidelines [4]. In patients who were treated prior to implementation of the updated image-guided adaptive BT (IGABT), high-risk clinical target volume (HR-CTV) definition, gross tumor volumes were excluded in the EQD2 analysis for HR-CTV and D90 calculations. During the study period, institutional practice was adapted to best incorporate the GEC ESTRO 3D image-based contouring guidelines. This was done on CT scan with the applicator in place, considering that MRI was not routinely available for each fraction. Radiology reports from CT, PET, and MRI (when available) were utilized to aid in delineation of the HR-CTV. Oncentra Treatment Planning System (version 4.3, Elekta) was utilized for all brachytherapy plans. In our institution, treatment planning procedure for hybrid Utrecht application begins with a customary tandem and ovoids treatment planning type process, with the dose normalized and optimized to applicator points and target HR-CTV points (resulting mostly in a pear-shaped dose distribution, except at the levels of interstitial needles). The resulting dose distributions are then reviewed on all slices (utilizing axial, sagittal, and coronal views) by the radiation oncologist and wherever necessary, the dose distributions are further refined by graphically changing local dwell times to modify isodose lines (Figure 2). This is done to achieve proper target coverage (D90), reduce hot spots, and to match patient anatomy while respecting OARs doses. A 6 or 7 Gy dose is prescribed to the 100% isodose line, while making every effort to limit the combined EBRT + BT OARs doses. Constraints include D2cc < 80 Gy to the bladder, and < 70 Gy to the rectum, sigmoid, and small bowel, equivalent dose in 2 Gy fractions (EQD2). In general, the tandem and ovoids contribution is around 80% of the dose, with interstitial needles contributing to 20% of the dose.
Fig. 1
Tandem and ovoids hybrid brachytherapy using Utrecht applicator. Shown are the tandem, ovoids, guiding tubes, and flexi-needles flushed with the ovoid surface

Fig. 2
Tandem and ovoids hybrid plan dose distribution and DVH. Top left – axial. Bottom left – coronal. Top right – sagittal. Bottom right – DVH analysis
Pre-brachytherapy MRI was utilized to assess treatment response to EBRT and to select patients for hybrid IC/IS BT, when available. The rate of reduction of tumor volume during BT was assessed by calculating the rate of change in HR-CTV from the CT acquired at each brachytherapy fraction.
Acute and late toxicities were graded according to CTCAE v. 5.0. Fistulas were classified once according to the anatomical site involved. If a fistula was due to tumor involvement, this was not scored as treatment-induced toxicity.
Treatment parameters tabulated included HR-CTV D90, V100, 0.1 cc, 1 cc, and 2 cc doses to OARs, including the bladder, rectum, bowel, and sigmoid. The D90 and D0.1cc, D1cc, and D2cc were recorded for each fraction and summed to a total BT-EQD2, which was then combined with the external beam EQD2 for the HR-CTV and OARs separately. These calculations were performed according to a linear quadratic formula assuming an α/β of 10 for tumor and 3 for OARs. Patient and tumor characteristics were analyzed, including FIGO staging (2009) [5], histology, pre-brachytherapy MRI tumor size, and the number of needles implanted. Median HR-CTV volume (mHR-CTV) was also queried and analyzed for association with outcomes, as was the rate of reduction of tumor volume during BT.
Statistical analyses
Bivariate associations of clinical and dosimetric variables with median HR-CTV volume were assessed using Wilcoxon rank-sum tests for continuous variables and χ2 tests for categorical variables. Kaplan-Meier method was used to estimate local control (LC), loco-regional control (LRC), distant control (DC), and overall survival (OS), with time-to-event data censored at the corresponding event or time of last contact within our health system. Cox proportional hazards regression was applied to analyze association with survival outcomes, and logistic regression was used to evaluate association with toxicity. All statistical analyses were performed in STATA v.14 (StataCorp, College Station, TX, USA).
Results
Seventy-one patients with locally advanced cervical tumors treated with hybrid tandem and ovoids IC/IS BT following definitive chemo-radiation were identified (Table 1). The median follow-up was 24.9 months, with a two-year LC of 83.6%. Two-year actuarial DC was 68%, LRC 72.0%, and OS was 88.6%. We observed 23 (32.4%) distant failures (DF), 12 (16.9%) local failures (LF), and 13 (18.3%) loco-regional failures (LRF). Six LF were identified as cervix only failures, without LRF or DF. Thirteen DF had no associated loco-regional or local failures. Eight patients with LRF had failure in the para-aortic lymph nodes, which were treated in the initial plan with extended field RT. Twelve patients with DF failed in the lungs, followed by the supraclavicular LNs (5) and the liver (4).
Table 1
Patient and tumor characteristics
The mHR-CTV D90 EQD2 was 87.4 Gy (IQR = 85.7-90.2) for all patients. FIGO IIB and IIIB patients had a mHR-CTV D90 EQD2 of 87.5 Gy (IQR = 86.3-91.2) and 86.8 Gy (IQR = 83.9-89.6), respectively. The median number of needles placed per patient was 2 (range, 1-6). Forty-six percent of FIGO IIIB patients and twenty-nine percent of FIGO IIB patients had a median number of needles per fraction > 2. The mean, median (range) needles per brachytherapy course for FIGO IB1/IB2, IIB, and IIIB were 8.8, 8 (range, 5-14); 10.2, 10 (range, 4-28); 11.6, 10.5 (range, 3-20), respectively. Pre-brachytherapy MRI reported tumor size was available for 26 patients, with a median of 2.2 cm in diameter. In total, thirty-five patients had a pre-brachytherapy MRI completed.
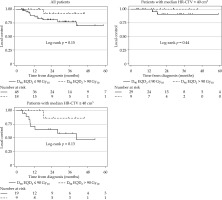
The mHR-CTV volume for all patients was 37.9 cm3 and the median V100 was 86.5%. The median mHR-CTV in patients with pre-brachytherapy MRI was 37.4 cm3 (range, 23.8-100.1), and for those without pre-brachytherapy MRI was 42.0 cm3 (range, 16.4-108.5). However, there was no difference in local control between these two groups (HR = 0.95, p = 0.93). A mHR-CTV volume of < 40 cm3 was significantly associated with improved LRC (HR = 3.52, 95% CI: 1.24-9.99, p = 0.018), and improved progression-free survival (PFS) (HR = 2.38, 95% CI: 1.14-4.95, p = 0.021) on univariable analysis (Table 2). This remained statistically significant on multivariable analysis (Table 3).
Table 2
Univariable analysis-clinical characteristics, dose and tumor volume metrics
Table 3
Multivariable analysis – clinical characteristics, dose and tumor volume metrics
Stage IIB patients with a mHR-CTV < 40 cm3 had significantly improved two-year LC as compared to a mHR-CTV of ≥ 40 cm3 at 100% and 71.8%, respectively (p = 0.019). Stage IIIB patients also demonstrated higher two-year LC, with a mHR-CTV of < 40 cm3 (85.1% vs. 70.7%) (p = 0.41). Two-year LRC for stage IIB patients with a mHR-CTV of < 40 cm3 was significantly higher than those with a mHR-CTV ≥ 40 cm3 at 100% and 56.5%, respectively (p = 0.001). However, two-year LRC for stage IIIB patients did not demonstrate this (66.7% vs. 60.6%) (p = 0.76). Two-year PFS rates for stage IIB patients with a tumor volume of < 40 and ≥ 40 cm3 were 77.8% and 45.7%, respectively (p = 0.051), and for stage IIIB patients were 66.7% and 40.4%, respectively (p = 0.18). The median tumor volume for stage IIB and IIIB was 37.9 cm3 (range, 21.8-74.3) and 35.5 cm3 (range, 16.4-108.5), respectively.
A HR-CTV D90 EQD2 > 90 Gy10 was associated with improved LC; however, it did not reach statistical significance (HR = 0.25, p = 0.19) (Figure 3). Total treatment package time > 56 days was significantly associated with worse loco-regional control (p = 0.047). A larger percent per day reduction in HR-CTV during BT was significantly associated with improved LRC (HR = 0.73, range, 0.54-0.99, p = 0.045) (Table 3).
Fig. 3
HR-CTV D90 and local control. Higher HR-CTV D90 demonstrates a trend towards improved local control in patients with large (median HR-CTV volume ≥ 40 cm3) and small tumors (< 40 cm3)
The median bladder 0.1 cc and 2 cc EQD2 doses were 102.9 Gy and 88.3 Gy, respectively (Table 4). The median rectum 0.1 cc and 2 cc EQD2 doses were 83.2 Gy and 70.0 Gy, respectively. Notably, the sigmoid 0.1 cc EQD2 dose was associated with late gastrointestinal (GI) toxicity, with a HR of 1.06 (95% CI: 1.01-1.10, p = 0.015) (Table 5). Bladder EQD2 doses were not associated with late genitourinary (GU) toxicity. Neither rectum nor bowel EQD2 doses were associated with late GI toxicity.
Table 4
Organs at risk doses from brachytherapy and external beam therapy combined, presented as equivalent dose in 2 Gy fractions based on α/β = 3
Table 5
Univariable logistic regression showing association between organs at risk doses and late genitourinary (GU) or gastrointestinal (GI) toxicities
Acute grade 2-3 GU toxicities occurred in 12.7% of the patients. There were no acute grade 4 or higher GU toxicities. Acute grade 2 GI toxicity occurred in 24% of the patients. There were no acute grade 3 or higher GI toxicities. One patient experienced a grade 2 vaginal hemorrhage, which occurred prior to starting EBRT, but recurred due to non-compliance with EBRT course. One patient experienced a grade 3 GU toxicity secondary to urosepsis during BT, which occurred due to a delay in percutaneous nephrostomy exchange. Another patient experienced an acute grade 3 vaginal hemorrhage, which occurred during the first week of EBRT.
Twelve percent of the patients experienced late grade 2 GU toxicity, and 1% suffered from late grade 3 GU toxicity. Twenty-one percent of the patients experienced late grade 2 GI toxicity, and 1% late grade 3 GI toxicity. There were no grade 5 toxicities. There were two treatment related fistulas, 1 rectovaginal fistula, and 1 vesicovaginal fistula. A rectovaginal fistula occurred in a patient with initial FIGO IIIB disease and rectal 2 cc EQD2 of 81.4 Gy. A vesicovaginal fistula occurred in a patient with initial FIGO IIIB disease and bladder 2 cc EQD2 of 95.9 Gy.
Discussion
We showed satisfactory clinical outcomes and modest toxicity in this large single-institution cohort of 71 patients, treated with hybrid tandem and ovoids IC/IS BT. Several applicators capable of both intracavitary and interstitial implantation have emerged, including tandem and ring, and tandem and ovoids-based designs. The Vienna applicator (Elekta, Veenendaal, The Netherlands; Varian, Palo Alto, USA) is a variation of a ring applicator modified with interstitial needle positions available through the ring [6]. A subsequent, recently described adjustment to this applicator allows for oblique needle placement through the ring of the initial Vienna applicator, named “the Vienna-II” [7]. The Utrecht applicator (Elekta) is an adaptation of the tandem and ovoids applicator, with interstitial parallel needle placement available via the channels in the ovoids [6]. In this study population, patients were treated with a tandem and ovoids applicator with interstitial needles delivered through the ovoid channels, using an image-guided BT approach. 3D contouring and treatment planning were performed, according to the published GEC-ESTRO guidelines, which define the HR-CTV or CTV-THR as to include the residual gross tumor as well as adjacent residual disease [4, 8, 9].
As evidenced in the initial reports of image-guided BT, the ability to deliver 87 Gy to the HR-CTV results in improved local control rates above 95% [10]. This was the first report to suggest that DVH parameters were important for outcomes prediction, and the American Brachytherapy Society (ABS), as well as, the GEC ESTRO currently recommend delivery of 80 Gy for complete or partially responding tumors < 4 cm at BT, with dose escalation to 85-90 Gy for non-responding tumors not meeting these criteria [4, 11]. The importance of dose coverage and optimization made possible by additional interstitial needle placement is therefore best demonstrated by its relation to HR-CTV D90. There is currently a lack of prospective randomized data; though, retrospective series provide compelling data for improved target coverage and clinical outcomes when using the hybrid IC/IS approach. Most notably, the RetroEMBRACE study analyzed patients from multiple centers and categorized them into two groups: one intracavitary and one IC/IS BT group. The data demonstrated an improved D90 to HR-CTV with systematic use of IC/IS BT. The reported D90 increased from 83 ±14 Gy to 92 ±13 Gy [12]. Furthermore, there was a significant improvement in local control of 10% for patients with HR-CTV volume ≥ 30 cm3, with no difference observed for those with HR-CTV < 30 cm3. Our results demonstrated that a HR-CTV D90 EQD2 > 90 Gy10 was associated with improved local control, although this did not reach statistical significance (HR = 0.25, p = 0.19).
A separate retrospective single-institution report of outcomes for IC/IS BT in Japan demonstrated good feasibility and a two-year local control rate of 80.2% [13]. The median HR-CTV volume at initial hybrid BT was 37.1 cm3, with a median HR-CTV D90 of 70.3 Gy and V100 of 95.9%. Recently, a North American series showed good feasibility of hybrid BT utilizing Vienna and Venezia devices, with a 12-month locoregional control of 80.6% [14].
Here, we demonstrated excellent feasibility and implementation of IGABT for LACC with the use of a hybrid tandem and ovoids applicator with holes for parallel interstitial needle implantation. Our patient population was comprised of mainly FIGO IIB and IIIB cervical cancer patients, with a median HR-CTV volume of 37.87 cm3. Forty percent of our patient population had a median HR-CTV of > 40 cm3 at the time of brachytherapy. Two-year LC and LRC for stage IIB patients with a mHR-CTV < 40 cm3 were significantly improved, as compared to ≥ 40 cm3 at 100% and 71.8%, respectively (p = 0.019), and 100% and 56.5%, respectively (p = 0.001). However, this trend was not statistically significant for stage IIIB patients. Therefore, confirming the importance of tumor dimensions at hybrid BT versus initial dimensions and FIGO stage group.
Patterns of failure observed in our cohort demonstrated a predisposition for distant failures over local or loco-regional failures. This supports findings that patterns of relapse for cervical cancer treated with IGABT have changed to more distant rather than local failures, as found in an analysis of the RetroEMBRACE cohorts [15]. OAR EQD2 doses were comparable with the published median D2cc doses to the bladder, rectum, and sigmoid in the RetroEMBRACE cohort. Our median bladder 2 cc dose was 88.3 Gy compared with 79 Gy, median rectum 2 cc dose was 70 Gy vs. 65 Gy, and median sigmoid 2 cc dose was 70.4 Gy vs. 65 Gy [12]. Of note, the sigmoid 0.1 cc EQD2 dose was associated with late GI toxicity, with HR of 1.06 (range, 1.01-1.10) (p = 0.015). Bladder EQD2 doses were not associated with late GU toxicity, and neither rectum nor bowel EQD2 doses were associated with late GI toxicity. In the most recent publication from the EMBRACE study group analyzing IC/IS technique, the mean bladder, rectum, and sigmoid D2cc doses for the tandem and ovoids IC/IS group were 79.4, 62.7, and 63.4 Gy, respectively [16]. Of note, our larger median HR-CTV volume of 37.87 cm3 may provide some explanation for the slightly higher OARs doses. Specifically, about 30% of the patients had a bladder EQD2 > 90 Gy. It is worth stating that our OAR dose estimation is based on a uniform dose concept as suggested in GEC ESTRO publications, and not optimized by identifying the most exposed volume of critical organs, as eloquently studied by Frohlich and colleagues [8, 17].
Although IC/IS applicators provide improved dose optimization and tumor control compared to IC applicators, it is not known whether the IC/IS approach provides equivalent outcomes when compared to a perineal interstitial implant for larger tumors. In a recent meta-analysis of published series on perineal interstitial BT performed using 3D image guidance, LC was reported at 79%, but ranged from 62-92% [18]. Sixty percent of patients had stage IIIB disease or higher, though local control or survival was not available on a per stage basis. A single-institution experience with interstitial BT for tumors larger than 30 cm3 demonstrated a two-year local control rate of 77.6% [19]. Of thirty-seven patients, 65% had stage IIIB disease or higher (49% stage III, 16% stage IV), and a median tumor volume of 59 cm3. Despite the large tumor volume, the median HR-CTV D90 was 87.4 Gy. Even having a large percentage of patients (40%) with high-volume disease, our clinical outcomes remained comparable to these published local control rates for tumors > 30 cm3 treated with an interstitial implant. We observed two-year local control rates in stage IIB patients of 71.8% and 100% for tumor volumes ≥ 40 and < 40 cm3, respectively (p = 0.019). In stage IIIB patients, the two-year local control rates were 70.7% and 85.1% for tumor volumes ≥ 40 and < 40 cm3, respectively (p = 0.41).
In an analysis of 85 cervical tumors treated with IGABT, MRI demonstrating infiltration of the inner, middle, and outer third of the parametrium at diagnosis resulted in a significantly increased risk for residual parametrial disease at brachytherapy (88% vs. 43%) [20]. The ability to cover this residual parametrial disease, in addition to consideration of the sidewall and vaginal extent, is the key for hybrid BT selection. Specific applicator selection and referral for brachytherapy may be impacted if initial imaging demonstrates concern for potential residual disease.
IC/IS BT can be performed with either a tandem and ovoids applicator with adaptation for parallel needle placement through the ovoids, or with a tandem and ring applicator with placement through the ring [3, 21]. Recently, a hybrid applicator capable of oblique interstitial needle placement, with an angle of 20 degrees relative to the tandem, was demonstrated to have adequate dose coverage of the distal parametrium and pelvic sidewall, showing local control rates of 76% and 72% at 3 and 5 years, respectively [7]. These data suggest that oblique interstitial implantation could represent an alternative to perineal interstitial implant for selected patients with distal residual tumor. In our series, we did not define the extent of involvement of the parametria or sidewall at BT, and therefore it is difficult to compare the outcomes. However, the reported HR-CTV volume across both centers in this series was 71 cm3 compared to our median HR-CTV of 37.9 cm3. Therefore, we likely had a less extensive cohort of tumors at the time of brachytherapy. Furthermore, at our institution, patients with distal extent of disease at BT are typically selected for interstitial implant.
Limitations of this study include its retrospective nature and the use of CT image-guided 3D-based planning, with the use of pre-brachytherapy MRI for response and implant guidance. Though, BT planning was done on CT, there is data to suggest that outcomes are similar when comparing CT- vs. MRI-based contouring, and is performed at other institutions without access to MRI-based simulation [19, 22]. Given the retrospective nature of our analysis, toxicity outcome comparisons may be limited due to observer’s bias. There is also a limitation due to short follow-up in a significant proportion of the patients. This is not uncommonly seen at our institution, where significant socio-economic factors have been shown to affect compliance on treatment, which may also contribute to follow-up [23].
Conclusions
Intracavitary/interstitial brachytherapy using a hybrid tandem and ovoids applicator for LACC provides satisfactory outcomes with excellent local control, overall survival, and modest toxicity profile. Dose coverage assessed by the HR-CTV D90 demonstrated a trend towards improved local control with higher dose. However, caution should be exercised when selecting patients for hybrid brachytherapy with a tumor volume ≥ 40 cm3, even though local control outcomes are comparable to published results for interstitial implant.



