Purpose
In rectal cancer, with the implementation of the total mesorectal excision (TME) and addition of neoadjuvant chemoradiotherapy (CRT), significant improvements have been reported in local control (LC) of approximately 5-10% [1,2,3,4,5,6]. Without a treatment, patients with local recurrence have a median survival of approximately 8 months only. Even after salvage treatment, 30-50% of patients will eventually die of locally recurrent disease [7,8].
Surgery is the most widely accepted standard curative salvage treatment option, and it has an acceptable quality of life (QoL). Complete surgical resection is associated with the best outcome in previously published series [9,10,11]. However, pelvic control after salvage surgery remains unsatisfactory between 40-50% [12,13]. The LC achieved is heavily dependent on additional therapies given with surgery. Use of intraoperative radiation therapy (IORT) resulted in improved LC and overall survival (OS) in recurrent colorectal cancer [14,15]. Surgery is just not always feasible, and to determine a treatment strategy for such patients is quite difficult.
Irradiation is another salvage treatment option for locally recurrent rectal cancer (LRRC), especially suitable for patients who have already received salvage resection, and who are medically and surgically inoperable. Irradiation with an external beam radiation therapy (EBRT) with or without concurrent use of chemotherapy can be considered, in general, as less invasive therapy than surgery. However, the total dose is generally limited to sub-curative doses because of dose limitation of surrounding organs such as small bowel, sigmoid colon, the rectum itself and bladder. Therefore, EBRT is considered to be a useful treatment just for symptom relief, but it has a small impact on long-term outcome [16].
In terms of optimizing radiotherapy effect, the tumor should receive a higher tumoricidal total dose while sparing the surrounding normal tissue to avoid severe toxicity. In this context, image-guided high-dose-rate interstitial brachytherapy (HDR-ISBT) might be a promising treatment option because it delivers a higher dose focally to the tumor while reducing the dose to organs at risk (OARs) in comparison to EBRT. There are only few publications in the literature on the application of HDR-ISBT for recurrent rectal cancer suggesting that it could be an effective alternative method for locally recurrent rectal cancer worth considering [17,18,19]. Here, we present a case report to illustrate the treatment effect of image-guided HDR-ISBT for LRRC after salvage surgery. Written informed consent was obtained from the patient.
Case description
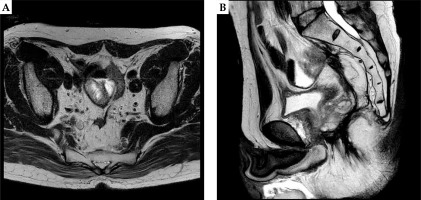
A 61-year-old male LRRC patient after salvage surgery who initially underwent laparoscopic high anterior resection (LAP-HAR) D3 regional lymph node dissection for primary rectal cancer (pT3N0M0, stage II, well-differentiated adenocarcinoma), with no adjuvant chemotherapy or radiotherapy was presented at our institute. Local recurrence was noted eight months after initial surgery, for which he underwent abdominoperineal resection (APR) as a salvage treatment, followed by adjuvant 8 cycles of XELOX (capecitabine and oxaliplatin) chemotherapy. Fifteen months post-salvage surgery, he developed local recurrence determined by positron emission tomography-computed tomography (PET-CT) measuring 23 × 25 mm close to the sigmoid colon in pelvis (Figure 1). Considering the third surgery as a technically challenging and intolerable, it was refused by the patient. The patient was referred to our department for the possibility of HDR-ISBT, and the combination of image-guided HDR-ISBT and EBRT was proposed.
Fig. 1
Axial (A) and sagittal (B) images of PET-CT images of recurrent rectal carcinoma in the presacral area closed to the sigmoid colon
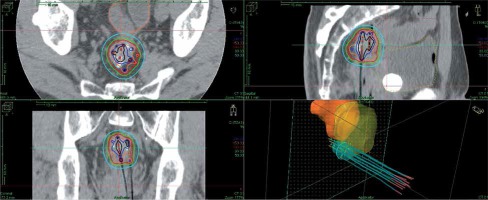
The technique of salvage image-guided HDR-ISBT for recurrent pelvic malignancies has been described elsewhere [20,21]. Eleven plastic 5F ProGuide® needles (Nucletron, an ELEKTA company, ELEKTA AB, Stockholm, Sweden) were inserted through a perineal Prostate Stepper Template® (Nucletron, an ELEKTA company, ELEKTA AB, Stockholm, Sweden) beyond the prostate. Trans-rectal ultrasound was not used in this case to guide the needle insertion, as the patient’s anus was closed during salvage APR. There was calcification inside of the recurrent tumor, which was visible with C-arm fluoroscopy. Initially, four needles were inserted toward the calcification based on fluoroscopy and trans-abdominal ultrasound guidance; then, CT was taken. It was found that one needle penetrated the tumor, and this needle was used as a landmark; the rest of the needle was inserted by fluoroscopy, CT, and trans-abdominal ultrasound guidance. After the needle insertion, a planning computed tomography was completed and dose calculation was performed using Oncentra® Brachy version 4.5.1 (Nucletron, an ELEKTA company, ELEKTA AB, Stockholm, Sweden). Clinical target volume (CTV) was contoured based on CT, and no planning treatment volume (PTV) margin was added since the needles moved with the tumor. Dose calculation was performed so that 100% isodose line covered the CTV. Prescribed dose per fraction was 6 Gy and the total prescribed dose was 30 Gy/5 fractions/3 days, applied twice-daily, with an inter-fractional interval of at least 6 hours. EBRT was performed after HDR-ISBT, with 39.6 Gy in 22 fractions. Figure 2 shows the isodose dose distribution of the HDR-ISBT. The sigmoid D2cc, bladder D2cc, and CTV D90 was 272 cGy, 212 cGy, and 840 cGy per fraction, respectively. Brachytherapy was carried out using an 192Ir remote after-loading system (RALS, MicroSelectron v2r® HDR Ir-192 source, Nucletron, an ELEKTA company, ELEKTA AB, Stockholm, Sweden).
Fig. 2
The isodose dose distribution of the interstitial brachytherapy. From a dosimetric point of view, the sigmoid D2cc was 272 cGy, bladder D2cc was 212 cGy, and CTV D90 was 840 cGy per fraction, respectively
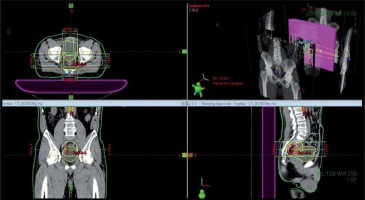
One week after HDR-ISBT, 39.6 Gy in 22 fractions of EBRT by 4-fields box technique was started. Figure 3 shows the isodose dose distribution of the EBRT. Radiotherapy-related toxicity was measured using the Radiation Therapy Oncology Group (RTOG) radiation morbidity scoring schema [22]. Post-treatment, the patient had grade 1 acute radiation-related diarrhea, and no late radiation-related toxicity of any grade was observed.
Fig. 3
The isodose dose distribution of the external beam radiation therapy with 4-fields box technique
Two months post-HDR-ISBT, MRI detected shrinkage of the recurrent pelvic tumor, and follow-up MRIs detected no tumor progression on five, eight, and fourteen months thereafter (Figure 4). The CEA level decreased sharply from 24.5 ng/ml before HDR-ISBT to 0.7 ng/ml 4 months after whole salvage radiotherapy, and the CEA level sustained within the normal range during follow-up until now.
Discussion
In rectal cancer patients, local pelvic relapse is one of the major failure patterns, which creates a major therapeutic dilemma. It can lead to a devastating condition such as intractable pain, pelvic infection, and bowel obstruction, which has a significant impact on the patient’s health-related QoL, and eventually ending up being a fatal condition [23]. The goals of treatment for locally relapsed rectal cancer are palliation of symptoms with a better QoL and if possible, achieving cure with low treatment-related complications. Therapeutic modalities such as repeated surgery, radiotherapy (EBRT, IORT, or intraoperative brachytherapy [IOBT]), chemotherapy, or combinations of these modalities can be proposed as salvage strategies, and the choice is dependent on the previous treatment and patient’s health condition as well as the balance between benefits and morbidity.
Surgery is often advocated as the most effective way to treat patients with LRRC. However, there is still a high incidence of a second local recurrence after repeated surgery of approximately 40-50% [12,13]. Additional therapies given along with surgery such as IORT could improve LC and OS in recurrent colorectal cancer [14,15]. Third time surgery is more aggressive and challenging, with higher morbidity and mortality rate than previous operations and tend to be refused by most of patients. Therefore, under such circumstances, radiotherapy or CRT is often the preferred salvage approach of treatment.
In terms of optimizing radiotherapy, the tumor should receive a higher tumoricidal total dose while sparing the surrounding normal tissue to avoid radiotherapy-related severe late toxicities. There are several articles, which proposed radiotherapy to be an optimal palliative procedure for recurrent rectal cancer [24,25,26,27,28]. Because of the poor perfusion in the recurrent tumor, LRRC is considered to be potentially radio-resistant. Since EBRT requires margins, which accounts for tumor/organ movement and set-up error, an application of high doses of EBRT is associated with a high-risk of radiation damage to surrounding normal structures such as gastrointestinal tracts or bladder [29,30]. Therefore, in general, only sub-curative doses can be delivered using EBRT and it is often used for palliative treatment. Because of the lower efficiency of EBRT, some novel radiotherapy techniques such as stereotactic body radiation therapy (SBRT), a proton or carbon-ion radiotherapy are under investigation for LRRC [31,32,33,34,35,36].
HDR-IBT is also a promising treatment option for LRRC; it delivers exceptionally high doses concentrated to the tumor compared to doses delivered with EBRT. Moreover, its steep dose gradient offers the protective property for surrounding critical OARs, which enables to safely deliver high tumoricidal dose to the target. It is expected that it can provide better clinical results than those offered by EBRT alone.
The use of brachytherapy in the treatment of LRRC was first reported in 1997 by Goes et al. [37]. Kolotas et al. [28] described HDR-ISBT as a valuable tool for delivering high doses and achieved effective symptom palliation in recurrent rectal carcinoma patients. As the radiation sources are implanted directly into the tumor in HDR-ISBT, the dose can be escalated to a level that is biologically lethal for all tumor cells. With the development of brachytherapy technology, image-guided HDR-ISBT is used in many institutions. Visualization of not only the CTV, but also the OARs by CT images integrated with a computerized system for implant planning and evaluation allows optimized dose distributions to be indexed based on individual anatomy [38]. Image-guided HDR-ISBT is used in many institutes for different tumor sites such as gynecologic, breast, or head and neck malignancies [39,40,41,42,43], but a lesser number of cases has been reported about CT-guided implantation of interstitial catheters in pelvis for the treatment of LRRC.
In our study, we have delivered image-guided HDR-ISBT followed by EBRT as a curative treatment for LRRC after failure from definitive and salvage surgery. An excellent clinical and biochemical results was noticed with a sharp decrease in CEA level from 24.5 ng/ml to 0.7 ng/ml in 4 months after implant. The tumor regressed considerably, as identified by MRI, two months after the whole salvage radiotherapy treatment, and no evidence of progression were noted until 14 months after radiotherapy. No radiation-related late toxicity was reported. The clinical and biochemical results of this salvage HDR-ISBT for LRRC are very encouraging, and a combination of EBRT with image-guided HDR-ISBT has the potential to be a valuable treatment option for this kind of patients.