Purpose
Esthetics and functionality are important considerations in the treatment of lip cancer. Brachytherapy (BT) is used alone or in combination with external radiation therapy for such patients. Low-dose-rate (LDR) BT is a well-established treatment, with a long history of good local control and esthetics [1, 2]. However, LDR-BT has limitations, such as radiation exposure to medical personnel and inadequate nursing care resulting from hospitalization of patients in a shielded hospital room. Furthermore, currently it is very difficult or impossible to obtain iridium-192 (192Ir) wires, nor most of the legislation of developed countries do not allow its use. On the other hand, interstitial high-dose-rate (HDR) BT has overcome these problems [3-15]. Old systems use two-dimensional images, such as orthogonal X-ray images for treatment planning [3, 4, 6, 9, 13]. However, this method does not allow radiation oncologists or medical physicists to identify tumors and organs at risk for dose delivery and evaluation. Three-dimensional imaging devices, such as computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and positron emission tomography (PET), are widely used, and three-dimensional tomographic images are now being integrated for HDR-BT of head and neck cancer, which is known as image-guided HDR-BT (IG HDR-BT) [16]. This technique enables the quantitative evaluation of the volume and dose delivered to the tumor and mandible, which are considered organs at risk. There have been several studies on IG HDR-BT for lip cancer [5, 7, 8, 10-12, 14, 15]. Six of them [5, 10-12, 14, 15] used interstitial applicators alone, and two [10, 12] performed both tumor and mandible dosimetry. Two studies employed both interstitial and surface applicators to improve dosimetry [7, 8]. One found that better dose distribution was achieved by using both interstitial applicators and molds with embedded applicators [8], but only the tumor dose was shown [8] and no data for mandibular dose were available in these two reports. Thus far, few studies have described both tumor and mandibular doses using IG HDR-BT with custom-made surface applicators as monotherapy.
The purpose of this study was to introduce the technique, and present the long-term clinical results and quantitative dose-volume evaluation of IG HDR-BT with custom-made surface applicators for the treatment of lower lip cancer as monotherapy.
Material and methods
Eligibility criteria for the study
Patients with localized lower lip cancer, who received IG HDR-BT with custom-made surface applicators as monotherapy at the NHO Osaka National Hospital between February 2012 and January 2015 were enrolled in this study. Both primary and recurrent cases were included.
Placement of interstitial and customized surface applicators
Gross tumor volume (GTV) was defined by visual inspection and palpation by at least two radiation oncologists. Holes were drilled in a pair of vinyl templates, where the interstitial and custom-made surface applicators were to be placed. The affected lip was positioned between the parallel templates (Figure 1A, B), and one to three interstitial applicators were placed in or around GTV through the holes in the templates in operating room under general or local anesthesia. Two to six surface applicators were arranged around the surface of GTV through the holes in the templates. Surface applicators were passed through the vinyl tube to avoid hyper-dose sleeves in the skin by maintaining a distance from the lip surface (Figure 1C). Two types of applicators were used: Single Leader® (Elekta AB, Stockholm, Sweden) or OncoSmart® catheter system (Elekta AB). An example of the application of the OncoSmart® catheter system is presented in Figure 2. The number of applicators applied for each patient is listed in Table 1.
Table 1
Treatment characteristics
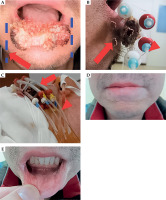
Fig. 1
Example of the placement of interstitial and custommade surface applicators, along with treatment results (case No. 1). A) Schematic diagram of two vinyl templates (blue dotted line) with holes (arrow) at the positions for interstitial and surface applicators. The affected lip was positioned between the parallel templates. B) Lateral view of the surface applicators supported by the vinyl templates. Holes were created for the interstitial applicators (arrow). Surface applicators (arrowheads) were placed to confirm the location before surgery. C) Placement of interstitial and surface applicators after surgery. Surface applicators (arrowhead) were passed through the vinyl tube (arrow) to avoid hyper-dose sleeves in the skin by maintaining a distance from the lip surface. D, E) One year and 10 months after brachytherapy. No evidence of disease was observed for the primary lesion, with slight hypopigmentation only
Treatment planning and dose delivery
A silicone replica of a lead shield was inserted between the gingiva and lower lip to avoid metal artifacts from CT. Medical physicists contoured the mandible as an organ at risk and performed applicator reconstruction on CT. Initially, a modified Paris system with computer optimization (geometrical optimization) was used for planning-aimed dose (PAD), which was the same as the prescription dose, i.e., to 85% of the basal dose using Oncentra® Brachy planning system, version 4.1 (Elekta AB). A radiation oncologists added a margin to GTV to contour clinical target volume (CTV). MRI was also used as a reference for CTV contouring in two cases (cases No. 4 and 5). Dose-volume histograms (DVH) were examined, and any inadequate values were manually corrected to ensure that the isodose curve of PAD adequately covered the CTV while preventing excessive doses to the mandible [17]. The dosimetric goal was set, so that the minimum dose that was administered to 90% of CTV would be greater than PAD. For new cases, 54 Gy was used, and 48 Gy was applied to recurrent patients for PAD, whereas the dose per fraction was 6 Gy. Irradiation was performed twice a day using MicroSelectron HDR® (Elekta AB), at least 6 hours apart. A lead shield was inserted between the gingiva and lower lip during irradiation to reduce the mandible dose, except for the edentulous patient (case No. 6). Patients were given gauze soaked in 2% lidocaine (Xylocaine® 2% jelly) to bite, to avoid unnecessary exposure to the upper lip and maxilla (Figure 2C).
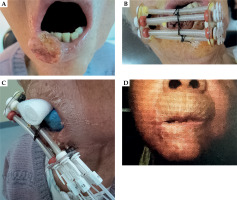
Fig. 2
Example of the placement of interstitial and custom-made surface applicators, along with treatment results (case No. 4): A) Photographs taken during the first visit. B) Placement of the interstitial and surface applicators using the OncoSmart® catheter system. C) Photographs taken during irradiation, showing the insertion of a lead block and gauze soaked in 2% lidocaine (Xylocaine® 2% jelly) into the oral cavity. D) Three years and four months after brachytherapy, with no evidence of disease observed at the primary lesion. However, hypopigmentation and telangiectasia were noted
Clinical endpoints and quantitative dose-volume evaluation
Clinical endpoints of this study were local control, regional control, and late adverse reactions. The latter included osteonecrosis of the mandible, skin hypopigmentation, telangiectasia, and other skin and subcutaneous tissue disorders, which were evaluated by the Common Terminology Criteria for Adverse Events version 4. Minimum percentage dose of PAD administered to 100%, 95%, and 90% of CTV (D100, D95, and D90, respectively), and percentage volume of CTV receiving 100% or more of PAD (V100) were calculated using DVH to evaluate the quality of treatment quantitatively. Minimum dose per fraction irradiated, i.e., 0.1 cm3, 1 cm3, and 2 cm3 volume of the mandible (D0.1cm3, D1cm3, and D2cm3) was determined.
Results
Six patients were enrolled in this study. Table 2 shows the characteristics of the patients. The median age was 79.5 (range, 46-95) years, and the male-to-female ratio was 3 : 3. Using the Union for International Cancer Control classification system (7th edition), 3, 2, and 1 patients were classified as T1N0M0, T2N0M0, and T3N0M0, respectively. All cases were histologically diagnosed as squamous cell carcinoma. One patient (case No. 4) was evaluated with a recurrent cancer with labial commissure invasion, for which the patient had previously undergone surgery and external radiation therapy to the primary site and cervical lymph nodes. Table 1 presents the treatment characteristics. CT (all cases) and MRI (2 cases: cases No. 4 and 5) were performed following the applicator placements for treatment planning. The median follow-up was 69.5 (range, 45-99) months (Table 1). The primary tumor and cervical lymph nodes were controlled in all patients. Three patients died of other causes (cases No. 2, 4, and 5). At the time of writing of this manuscript, disease-specific survival remains 100%. The 95% confidence interval (95% CI) for the observed disease-specific survival rate of 100% was ranging 54-100%.
Table 2
Patient characteristics
Late adverse reactions, including skin hypopigmentation were observed in three cases (at 16: case No. 1; at 40: case No. 4; and at 95: case No. 6 months post-treatment), telangiectasia in four cases (at 57: case No. 2; at 70: case No. 3; at 40: case No. 4; and at 95: case No. 6 months post-treatment), and other skin and subcutaneous tissue disorders (mucosal roughness) in one patient (case No. 5 at 31 months post-treatment). All of these were grade 1, and no late adverse reactions of grade 2 or worse were observed (Figure 1D, E). The median values of D100, D95, D90, and V100 were 91.2 (range, 60.2-113.2) %PAD, 106.3 (range, 78.3-128.4) %PAD, 108.9 (range, 88.6-134.7) %PAD, and 99.3 (range, 84-100) %CTV, respectively. The median values for D0.1cm3, D1cm3, and D2cm3 of the mandible were 3.2 (1.5-4.1) Gy/fraction, 2.3 (range, 1.1-2.8) Gy/fraction, and 2 (range, 1-2.5) Gy/fraction, respectively (Table 3). An example of dose distribution curve is shown in Figure 3.
Table 3
Quantitative dose-volume evaluation
[i] CTV – clinical target volume, PAD – planning-aimed dose, DX (%PAD) – minimum percentage dose of PAD that was administered to X% of CTV, V100 (%CTV) – percentage volume of CTV receiving 100% or more of PAD; DXcm3 (Gy) – minimum dose per fraction irradiated of X cm3 volume of the mandible, fr. – fraction
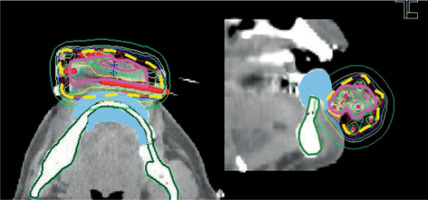
Fig. 3
Example of a dose distribution curve (case No. 1). The pink, yellow-dotted, and green lines represent the clinical target volume, 6 Gy isodose curve (as planning-aimed dose per fraction), and the mandible, respectively. The light blue area shows a silicone replica inserted instead of a lead shield to avoid metal artifacts for computed tomography
Discussion
Previous studies utilizing HDR-BT as a treatment for lip cancer have often combined surgery and/or external radiation therapy [3, 5, 7, 9, 11, 15]; however, when used in combination with surgery, esthetics can be compromised. In addition, long-term xerostomia or a decrease in taste function occurs less frequently with monotherapy [18]; therefore, it is preferred whenever possible. There are only four papers, including this study, reporting the treatment outcomes of lip cancer using IG HDR-BT as monotherapy (Table 4). The number of patients in these studies is small, but all have shown good clinical results without serious late adverse reactions. There have been other studies on HDR-BT as a monotherapy for lip cancer. Tagliaferri et al. evaluated HDR-BT alone in patients with cancer of the maxillofacial region, including 10 patients with lip cancer. The outcomes for lip cancer patients were excellent. However, there was no concomitant use of surface molds, nor data on DVH. Additionally, the follow-up period was shorter than ours [14]. Similarly, Tuček et al. investigated 29 patients with lip cancer treated with HDR-BT alone, achieving excellent results. However, the overall reporting included its use as an adjuvant treatment, and there was no utilization of surface molds and no report on the mandibular dose [11]. Feldman et al. [8] and Masui et al. [10] showed clinical outcomes and DVH parameters in three and six patients, respectively. Feldman et al. [8] described three cases of IG HDR-BT alone for lower lip cancer using a combination of interstitial sleeve applicators and a surface mold with embedded sleeve applicators, but reported tumor doses only and mandibular doses are unknown [8]. On the other hand, Masui et al. quantitatively demonstrated both tumor and mandibular doses, whereas they placed applicators only interstitially in the tissue [10]. Additionally, Torres-Quispe reported tumor and mandibular doses, but only for one case and without the use of surface molds [12]. The cases in the present study were followed for a longer period, with quantitative dose-volume evaluation of the tumor and mandible. In all cases, the applicators were placed not only through the tissue, but also on the tumor surface. Although this study includes a small cohort of patients (six cases), it may be considered a useful reference for future considerations.
Table 4
Studies on lip cancer treatment using IG HDR-BT as monotherapy
| Author (year) | n | T category | Planning | DVH (median) | Applicator placement | Follow-up (months) | LS or spacer | LC (%) | Toxicity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V100 (%CTV) | D90 (%PAD) | D0.1cm3 (Gy/fr., mandible) | |||||||||
| Feldman (2014) [8] | 3 | 3T2 | 3D | 91.7 | N.A. | N.A. | Interstitial + surface | 29, 32, 38 | 〇 | 100.0 | * |
| Masui (2020) [10] | 6 | 6T2 | 3D | N.A. | 133.0 | 1.65 | Interstitial | 13 (median) | 〇 | 100.0 | No grade 2 or worse |
| Torres-Quispe (2021) [12] | 1 | 1T2 | 3D | 13.0*** | 90.0 | 0.7895** | Interstitial | 24 | 〇 | 100.0 | No serious complications |
| This study | 6 | 3T1 2T2 1T3 | 3D | 99.3 | 109.0 | 3.2 | Interstitial + surface | 69.5 (median) | 〇 | 100.0 | No grade 2 or worse |
[i] HDR – high-dose-rate, DVH – dose-volume histogram, CTV – clinical target volume, PAD – planning-aimed dose, V100 (%CTV) – percentage volume of CTV receiving 100% or more of PAD, D90 (%PAD) – minimum percentage dose of PAD that was administered to 90% of CTV, fr. – fraction, LS – lead shield, LC – local control, 3D – three dimension, N.A. – not available, * minimal fibrosis and dry lips, ** there is no mention of whether it refers to D0.1cm3, *** %planning target volume (= CTV + 1 cm)
The importance of using custom-made surface applicators for IG HDR-BT treatment of lip cancer is twofold. One is to deliver a sufficient dose to the tumor protruding from the lip, and the second is to prevent edema associated with the penetration of applicators into the tissue. In the case of a large tumor, such as T3 (case 1, Figures 1, 2) in our study, custom-made surface applicators were effective in delivering a sufficient dose to the tumor, which also followed the principle of the Paris system by surrounding the tumor with a radioactive source. However, if the surface applicator is placed too close to the lip surface, the skin surface may experience hyper-dose around the sleeves in the skin, which may cause ulcers. Therefore, we placed the superficial applicator on the tumor surface after it was inserted into the vinyl tube (Figure 1C). In this way, the vinyl tube acts as a spacer to maintain distance between the applicator and the skin, thus preventing hyper-dosing around the sleeves.
In a study by Yoshida et al., a case with applicator-induced edema during IG HDR-BT in tongue cancer was shown [19]. They illustrated that when unexpected tongue edema occurs, the protruding area is underdosed, which results in tumor recurrence. Schultze et al. investigated the incidence and extent of edema in 51 patients, who primarily suffered from base of the tongue cancer, in which the median width of edema was 6.0 mm, as measured by MRI [20]. To prevent underdosing due to edema, it is advisable to have a device that counters edema-related tissue protrusion outside of the target area [19]. The lips are a susceptible location for idiopathic edema, such as Quincke’s edema [21], in addition to applicator-induced edema. Therefore, custom-made surface applicators are very important tools to prevent edema during IG HDR-BT for lip cancer.
Only one study has reported clinical outcomes and dose-volume evaluation of IG HDR-BT with customized surface applicators as monotherapy for lip cancer (Table 4). Feldman et al. reported three cases of IG HDR-BT alone for lower lip cancer, using a combination of interstitial sleeve applicators and a surface mold with embedded sleeve applicators [8]. They placed one to four applicators in the tumor, and one to three in the mold, with V100 ranging from 89-93.81%. After 29-38 months of follow-up, the patients were locally controlled, with excellent esthetic and functional outcomes. They concluded that the technique combining interstitial applicators with a surface mold and embedded applicators, resulted in superior tumor coverage and conformality [8]. In the present study, it was not possible to compare dose distributions with and without surface applicators; however, we demonstrated a better median V100 of 99.3% CTV, and good clinical results over a longer follow-up period (range, 45-99 months).
The earlier GEC-ESTRO recommendations [22, 23] do not provide a dosing schedule for IG HDR-BT as monotherapy, specifically in lip cancer. In a recent article [24], the recommended doses for lip cancer are as follows: 3 Gy × 18 fractions, 3.5 Gy × 14 fractions, 4 Gy × 13 fractions, and 4.5 Gy × 9 fractions for small tumors and post-operative cases, and 5 Gy × 9 fractions for larger tumors. Therefore, many dose schedules are recommended. We applied the IG HDR-BT parameters for tongue cancer, as published by Yoshida et al. [16], who included a dose of 54 Gy in 9 fractions in the absence of prior irradiation, and one dose reduction to 48 Gy in 8 fractions in the event of prior irradiation.
A serious late adverse reaction of oral cancer in HDR-BT is mandibular osteonecrosis [25]. In HDR-BT for lip cancer, Guinot et al. treated 104 patients and reported no cases of osteonecrosis [7]. They utilized L-shaped lead sheets to reduce radiation exposure to the opposite lip, tongue, and underlying mandible. Merfeld et al. investigated 10 patients with lip cancer treated with IG HDR-BT alone, and no cases of osteonecrosis were observed. Additionally, they used a custom-made mouthpiece for normal tissue protection [15]. Based on the GEC-ESTRO recommendations, a protective device is mandatory for the mandible, and a lead shield may be used to protect the mandible [22]. In this study, we utilized a lead shield in all but one edentulous patient. Masui et al. described a custom-made protective device in collaboration with a dental practice [10]. Cooperation with dentistry may be particularly important for edentulous patients, who present difficulty with inserting a standard lead shield. There is only one study on mandibular doses in IG HDR-BT for lip cancer as monotherapy. Masui et al. [10] reported a median D0.1cm3 of 1.65 Gy/fraction in six lower lip cases, whereas it was 3.19 Gy/fraction in the present study. Both reports (except for the edentulous patient in our study) used a lead shield during irradiation, so the actual dose may be lower compared with that mentioned above, because inhomogeneity correction calculations were not performed in either study. Akiyama et al. demonstrated the effect of a lead shield during IG HDR-BT for mobile tongue cancer [26]. Using a lead thickness of 4 mm, D0.1cm3 was reduced to approximately half. Even in the case of lip cancer, the actual D0.1cm3 maybe half of the reported value.
No evidence of local recurrence, nor late adverse reactions, such as osteoradionecrosis after long follow-up suggests that the tumor dose reported in the current study is sufficient, and that the mandibular dose was below the tolerated dose (Table 3). Among our patients, cases No. 4 and 5 had lower CTV doses than those of the other four cases. In both patients, the GTV dose was sufficient (e.g., V100 (%GTV) = 100% (case No. 4)). The reason for the lower CTV dose was that applying a sufficient dose to the CTV resulted in an excessive mandibular dose.
One limitation of this study is the small number of patients. Further studies with larger cohorts are needed in the future.



