Purpose
Prostate cancer (PCa) is the second most common cancer, and the fifth leading cause of death from cancer in men in the world [1]. The implementation of prostate specific antigen (PSA) and increase of public awareness for screening in the early 2000s, led to an age decrease at diagnosis and pathological stage, which raises the question of young patients care [2]. Life expectancy of men aged 40 is about 40 years, and is still 22 years for men aged 60.
The main goals of treatment today are to achieve complete healing with as few side effects as possible. Brachytherapy (BT) is an effective treatment for PCa.
If all available treatments have excellent progression-free survival, side effects are analyzed to guide the best choice of treatment [3]. Brachytherapy offers lower side effects in erectile function (EF) and continence than other radical treatments [4, 5].
Brachytherapy recommendations for young men (YM) are based on two studies [2, 6]. In the first one, the biochemical recurrence-free survival (BRFS) of 108 patients aged 54 or less was 96% at 8 years [2]. In this study, 43% of patients had complementary external beam radiation therapy (EBRT) at baseline. There was no functional outcome assessment. In the second study, 1,665 patients were assessed (median follow-up of 68 months), and BRFS (Phoenix criterion) was 93% and 87% for patients over 60 years old at 5 and 8 years vs. 95% and 92% for younger patients, respectively (n = 367; p = 0.07) [6]. Definition for BRFS was the same as currently used, but 33.3% of patients had also EBRT, 55% additional hormone therapy (HT), and 21.7% had high-risk PCa.
In 2014, Tétreault-Laflamme et al. have defined an innovative index named ‘Quadrella’ specific to BT, associating all the four parameters of interest to assess oncological and functional outcomes after BT, including BRFS, EF, evaluation of the urinary toxicity, and rectal disorders [7]. In a study by Coquet et al. from our center, the results of Quadrella over 3 years were studied. The definition used to specify urinary toxicity was Crook positive index (i.e., IPSS > 15 and ΔIPSS ≥ 5). In this study, 100 patients, with mean age of 63.9 years (range, 50-77 years) were analyzed for 3 years post-BT, and 46.7-61% achieved the Quadrella objective (clinical condition close to the pre-implant status) [8]. However, the results were not analyzed according to age at diagnosis, and it may be hypothesized that they could be even better in young patients.
Low-dose-rate (LDR) BT has proven its effectiveness from an oncological point of view in YM. It must now be demonstrated that functional preservation is its asset. No study has ever compared both oncological and functional results in YM treated with exclusive LDR-BT in low-risk or favorable intermediate-risk (LR-FIR) PCa. In this setting, we aimed to study the impact of age on Quadrella index in patients aged 60 years or less by comparing them to older patients.
Material and methods
Study design and inclusion criteria
The present study was a mono-centric retrospective cohort type with prospective assessment. From June, 2007 to June, 2017, 463 patients with localized PCa were treated with LDR, iodine-125 (125I) BT, and had a minimum follow-up of 2 years. Inclusion criteria followed BT eligibility according to European and American guidelines [9]: 1) LR-FIR PCa according to D’Amico classification, i.e., Gleason score of < 7 with PSA < 10 ng/ml and < T2b, or only one of the following criteria: PSA 10-15 ng/ml or T2b or Gleason 7 (3 + 4); 2) Less than half of positive biopsies; 3) Initial prostate volume < 50 cc, or 80 cc decreased to 50 cc after 3 months of pre-operative hormonotherapy; 4) Pre-operative IIEF-5 > 16 (International Index of EF: 5 items) without any drug assistance. The IIEF-5 is a validated self-administrated questionnaire that allows to classify EF in different classes according to the score obtained: no erectile dysfunction (ED): > 21; weak ED: 21-17; weak to moderate ED: 16-12; moderate ED: 11-8; severe ED: < 8. Exclusion criteria were: 1) Pre-operative IIEF-5 ≤ 16; 2) History of pelvic EBRT; 3) Intake of phosphodiesterase inhibitor 5 (PDE5i) before BT. Patients were not randomized and treatment was selected after clear, fair, and appropriate information.
Of the 463 patients treated by BT, 143 had too low IIEF-5, 14 had no sexual intercourse, sexual function was not evaluated in 25 patients, and 59 patients were excluded because they had minimal channel prostate photo-vaporisation before BT. Therefore, 222 patients with localized PCa displaying initial IIEF-5 score of > 16 were included. All patients were sexually active at baseline. Population characteristics are summarized in Table 1. There was no difference in tumor characteristics: pre-operative PSA (≤ 10 and 10-15 ng/ml), ISUP score, D’Amico classification and cT stages). No patient received EBRT and 8.9% (n = 20) had 3 months of pre-operative HT because of prostate volume > 50 cc.
Table 1
Population characteristics
Brachytherapy technique and assessment of Quadrella index
All BT were performed by the same experienced team including an urologist, a radiation therapist, and a physicist. The technique using real-time 125I was carried out with a prescribed dose of 160 Gy [10, 11]. Per-operative and post-operative dosimetry characteristics are summarized in Table 2. To assess oncological and functional results of BT, Quadrella composite index was applied [7]. The Quadrella index was achieved after BT in case of: 1) Absence of biological recurrence (Phoenix definition: PSA < nadir +2); 2) IIEF-5 > 16 (with or without PDE5i); 3) No significant urinary toxicity (International Prostate Symptom Score) IPSS < 15 or IPSS > 15 and ΔIPSS < 5 according to Crook et al. [10]; 4) No rectal toxicity (Radiation Therapy Oncology Group, RTOG = 0). These questionnaires were completed by all included patients before BT (or before HT), and then annually. The primary endpoint was the rate of patients achieving the Quadrella index in each group according to age at BT, i.e., < 60 vs. > 60 years.
Table 2
Modalities for prostate brachytherapy
[i] D90 – dose received by 90% of prostatic volume, V100 – prostatic volume receiving 100% of prescribed dose of 160 Gy, D2cc – maximal dose received by 2 cc of rectal volume; D0.1cc – maximal dose received by 0.1 cc of rectal volume, R100 – dose received by 100% of rectal volume, D10 and D30 – dose received by 10% and 30%, respectively, of urethral volume
Statistical analysis
Student’s t-test and Mann-Whitney test were applied to analyze statistical differences between the 2 groups, and to compare means and binary data, such as frequency analysis. An exact Fisher test was used at 1 year, 2-year, 3-year, 4-year, 5-year, and 6-year follow-up after BT. Value of p < 0.05 was considered statistically significant.
Results
A population of 222 patients was analyzed. The proportion of patients studied corresponded to patients who were not lost to follow-up, and for whom we had sufficient inside, ranging 97.1-98% at year 1 to 74.7-76.6% at year 5 and 62.8-69.2% at year 6 (Table 3). Patients were considered not evaluable when they did not have sexual activity for other reasons than ED (non-urological disease, no partner, or partner condition not allowing intercourse), and lastly when they died from non-cancer-related causes. The rate of evaluable patients ranged from 88.2-95.7% of the theoretic patients at year 1 to 51.6%-74.5% at year 5 and 44.9-69.2% at year 6 (Table 3).
Table 3
Proportion of patients validating the Quadrella index by year of follow-up care and according to age (≤ 60 years vs. > 60 years)
Quadrella outcomes
Within the first 3 years, 77.6-95.7% of the overall population was evaluable, while 69.2-79.6% remained evaluable in the 3 subsequent years in YM, and only 45-61% in the older patients (Table 3). Only about 25% were lost to follow-up at 5 years and 30-38% at 6 years. Nevertheless, among the patients studied and evaluable, the proportion validating the Quadrella was significantly higher from year 2 in men aged ≤ 60 years (Table 3). This rate increased in YM from 40.3% to 81.5% from year 1 to 6, while for the older group, 33.6% at year 1 was observed and remained relatively stable thereafter (40-46.5%) (Table 3).
Oncological outcomes
Overall, after six years of follow-up, eight patients (3.6%) presented a PCa recurrence and all were aged above 60 years at baseline. One YM had a PSA level above nadir +2 at year 2, but it was a PSA bounce. Of the 8 patients, 7 were metastatic, one of whom died after six years and one had a local recurrence. At year 5, no biological recurrence was observed in 100% and 91.8% in evaluable patients ≤ 60 years old vs. > 60 years old, respectively (p = 0.29) (Table 4). At year 6, only 8.6% (3/35) of the evaluable patients in the older group presented disease progression (Table 4).
Table 4
Causes of the Quadrella failure by year of follow-up according to age
At 6 years, BRFS was 100% and 94.7% for patients ≤ 60 years and > 60 years, respectively. Specific survival (SS) was 99.3% and overall survival (OS) was 100% and 98% in the YM and the older group, respectively.
Toxicity outcomes
We did not observe any significant difference in the proportion of patients treated by PDE5i: 20.9-33.3% in YM vs. 18.6-24.4% (p = 0.3-0.7) (data not shown). PDE5i intake increased from 20% in the first year to 30-33% thereafter in YM, but remained relatively stable in older patients, with about 20-25%. The criterion of efficient EF (IIEF-5 > 16) largely explained Quadrella failure (Figures 1, 2). Erectile function criterion was validated (with or without PDE5i) by 67.2-81.4% of YM, while only by 40-56.1% of older patients, with a significant difference starting from year 4 in favor of YM (Table 4). Mean IIEF-5 decreased from 22 pre-operatively to 19 in YM vs. 21 to 15 in older patients (p = 0.01). The rate of patients maintaining a good EF (IIEF-5 > 16) was 67.2% in the young group at 1 year vs. 49.3% in the older group (p = 0.03), and remained relatively stable with time showing nearly 70-80% for YM, but decreased progressively in older patients from 57.9% at year 2 until 42.4% at year 6 (Table 4, Figures 1, 2). Severe ED (IIEF-5 < 12) was observed in only 4.7-13.4% in YM compared with 16.5-30.3% in older patients. Significant difference was reached at year 4, showing 4.7% vs. 21.2% (p = 0.02), and 26.7% of these patients did not accept PDE5i (data not shown).
Fig. 1
Proportion of patients ≤ 60 years old who did not validate each Quadrella criterion by year of follow-up
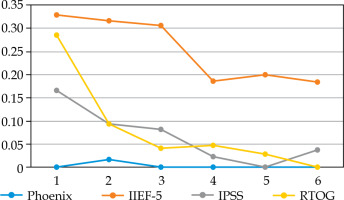
Fig. 2
Proportion of patients > 60 years old who did not validate each Quadrella criterion by year of follow-up
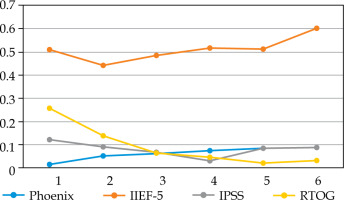
Significant urinary disorders (positive Crook index) were observed mainly in the first 2 years (about 9-16%) and decreased thereafter (0-8.6%), with no significant difference according to age except the fifth year (Table 4). From the second year, at least 90% of patients did not display any significant urinary symptoms. Mean IPSS increased from 4.5-4.8 at baseline to 8.6-8.7 at 1 year, 7.4-7.7 at 2 years, and then remained stable with nearly 6 until year 5. At 6 years, a slightly not significant difference was observed in mean IPSS: 5.25 in YM vs. 6.5. Severe LUTS (low urinary tract symptoms) (i.e., IPSS > 20) at any time of the follow-up was observed in 6/70 (8.6%) and 8/152 (5.3%) in young and older patients, respectively (p = 0.56). Urinary events, including urinary retention with necessary permanent drainage, TURP, bladder neck incision, urethral dilatation were very infrequent: 4/70 (5.7%) and 12/152 (7.9%) (p = 0.79), leading to observed urinary event-free survival in 94-97% and 90-94% of patients ≤ 60 years vs. > 60 years.
In the same way, rectal toxicity was observed mainly in the first 2 years (9-28%) without any difference in the two groups (Table 4). After the second year, rectal toxicity (RTOG > 1) was noted in less than 5%. Finally, 44/70 (63%) and 102/152 (67%) patients did not demonstrate any rectal toxicity during the 6 years in the young and older groups, respectively. In addition, after two years of follow-up, more than 90% of patients in both the groups had neither urinary nor rectal toxicity, with no significant difference between the two age groups.
Discussion
As the Trifecta, which is widely used to assess oncological and functional outcomes after radical prostatectomy, the Quadrella index has been defined by Tétreault-Laflamme et al. in order to specifically evaluate oncological and functional results after BT, including urinary and erectile disorders as well as rectal symptoms [7]. In this way, about 50-60% of patients validated the Quadrella in the 7 years following BT as Tétreault-Laflamme et al. and our group published [7, 8]. To the best of our knowledge, the present study is the first reporting the results of Quadrella according to age, which can be consequential when counselling patients about curative treatment of PCa in the setting of BT. Certainly, our and Tétreault-Laflamme et al. studies, it was shown that in the general population the results might be even better in YM. Moreover, in the assessment of functional outcomes, we used validated self-administrated questionnaires prospectively recorded, and all patients at baseline had normal or slightly affected EF (IIEF-5 > 16). As a lot of studies on Trifecta in RP presented results at 2 years, and as urinary and rectal toxicities post-BT may be observed early in the evolution [11, 12], we decided to assess the Quadrella also in the first 2 years. In this way, ≥ 75% the first 5 years was observed, with 58.7-81.5% reaching the Quadrella from year 2-6 in the YM group compared with 41.5-46.5% in the older group (p ≤ 0.05). The main cause of failure was ED. However, 67.2-81.5% of YM maintained a good EF (IIEF-5 > 16) compared with 40-56% in the older group. Oncological failure was not frequent, 1.6% vs. 8.6% in patients aged ≤ 60 vs. > 60 years old, respectively (not significant difference). In addition, about 88-93% of patients who failed the Quadrella had only one kind of toxicity. No patient had the 3 associated toxicities.
Several studies have focused on the oncological results of BT in young patients, but none has strictly compared the functional results. Regarding other BT series, Langley et al. observed excellent oncological results of BT in YM [13]. In this largest available series including 3,262 patients, of whom 597 men were ≤ 60 years, 316 patients presented low-risk cancer. At 10 years, the authors observed 95% of BRFS, 98% of overall survival, and 99% of specific survival. In our study at 6 years, 100% and 94.7% of BRFS, 100% and 98% of overall survival, 100% and 99.3% of specific survival, respectively, were observed for patients aged ≤ 60 and > 60 years old. In a study, Winoker et al. assessed the long-term results of BT, and used a projection by Kaplan-Meier in evaluating 423 young patients (≤ 60 years) [5]. They observed 89% and 88% of BRFS patients (Phoenix criterion) at 10 and 15 years, respectively. As in other studies, the population was not entirely comparable to ours, because 22% had high-risk PCa and 47.5% received a combined treatment: BT + EBRT, or BT + HT, or BT + EBRT + HT. Therefore, the current study retains its relevance for the oncological evaluation of exclusive BT. In a Buckstein et al. study, among 131 patients, with median follow-up of 11.5 years, the authors observed 13 biological recurrences (9.9%), 5 metastatic recurrences, and one PCa-related death [14].
Concerning EF, Cesaretti et al., as in our study, observed a link between age at BT and EF [15]. Indeed, of the 223 males they studied, 131 (59%) had optimal EF before BT. In the last evaluation, 51 patients (40%) had good EF with IPDE-5 (44.9%), yohimbine (n = 2, 4%), or alprostadil (n = 5, 10%). The authors used Mount Sinai erectile function (MSEF) scoring system to assess ED. A score of < 2 reflected a significant ED (rigidity, not allowing penetration). Indeed, age at implantation was predictive of EF after more than 7 years of follow-up; 23/25 (92%) of men aged 50-59 years had MSEF score of ≥ 2, while 48/75 (64%) of patients aged 60-69 years old, and 18/31 (58%) of patients aged 70-78 years (p = 0.01). Moreover, the authors observed a difference when IIEF-5 score of ≥ 16 was applied, with 16/25 (64%) in the group aged 50-59 years, 20/75 (27%) in 60-69 years old patients, and lastly 6/31 (19%) in the older group (70-78 years) (p < 0.001). In our study, there was also a significant difference at 6 years concerning IIEF-5 score of > 16, i.e., 81.5% (22/27) in patients ≤ 60 years vs. 40% (14/35) after 60 years (p = 0.0001). The Mount Sinai study also showed that, according to the score used, the rate of patients without ED may be different, but there was a correlation between the scores. IIEF-5 scale had poorer results compared to MSEF scale (MSEF score of ≥ 2), probably due to a more stringent definition of ED. A study carried out in our center by Schoentgen et al. did not show any significant difference for IIEF-5 > 16 at 1, 2, 3, and 4 years after BT, with nearly 54-64% [16]. In other words, after a decrease in post-operative IIEF-5, IIEF-5 remained stable over time. Our two studies by analogy with EBRT seem to support the hypothesis that ED is secondary to vascular structures damage of the penile bulb. Certainly, as for EBRT, after a decrease of IIEF-5 at years 1, 2, the IIEF-5 score seems to stabilize thereafter, and the toxicity when present seems progressive [17-19]. In addition, we observed that the main cause of Quadrella failure was sexual toxicity, ranging about 18-33% in YM compared with 44-60% in older patients, while urinary toxicity was observed in less than 16.5% and rectal toxicity in less than 14% after the first year. It is noted that among patients aged > 60 years, urinary and sexual toxicities after decreasing until year 2 (EF) or year 4 (urinary status), tended to increase. They were probably not related to BT but to the increased prevalence of this type of dysfunction with age.
Considering other functional outcomes, Kollmeier et al. assessed urinary toxicity by analyzing frequency of acute urinary retention (AUR) [20]. During follow-up, of the 1,665 men, 141 had AUR at some point, including 26/378 YM (7%) and 115/1,287 older men (9%) (p = 0.247), which is more common than observed in our study, with 2/70 (2.9%) and 10/152 (6.6%) in young and older patients, respectively. Rectal toxicity (RTOG ≥ 1) was observed mainly in the first 2 years (9-28%) without any difference in the 2 groups (Table 4). After the second year, rectal toxicity was observed in less than 5% of patients, and this is in line with our and other previously published studies on Quadrella [7, 8]. In addition, after two years of follow-up, more than 90% of patients in both the groups had neither urinary nor rectal toxicities (no significant difference between age groups).
Considering oncological and functional results of PCa curative treatment, the first composite index published called ‘Trifecta’ for RP was defined by Salomon et al. in 2003 that allows studying together all the parameters involved in the success of operation, such as cancer control, continence, and EF [21]. This score has been assessed in many studies, and usually the rate of patients achieving the Trifecta ranges from 20% to 72% at 1 year, and 40% to 60% at 2 years [22]. The differences observed were mainly due to a high variability of definitions concerning potency and continence [12]. As we stated in a previous publication, the Quadrella rates were about 60% at 2 and 3 years, and in the present study compared favorably with Trifecta rates of several series of RP using validated questionnaires for ED [8, 22].
Several limitations to the current study should be addressed. First, the retrospective design introduces the potential for selection bias. However, the questionnaires were given prospectively. Secondly, several definitions of ED may be applied, which can impact the results. In 2009, in a meta-analysis, Tal et al. found 22 different definitions of satisfactory EF [23]. In most studies, EF was considered satisfactory if patients could have erections sufficient for penetration, without considering frequency nor difficulties in maintaining erection. However, the IIEF-5 is a validated questionnaire. Also, the lack of matching in the study could generate a selection bias. Nevertheless, the two studied populations were initially comparable regarding the oncological parameters and the rate of HT treatment (to decrease volume). Also, all patients were selected at baseline, with IIEF-5 score of > 16, although the ratio of IIEF-5 between 21 and 25 was significantly higher in young patients. Despite these limitations, we believe that the results of our investigation provide useful information on assessing oncological and functional results of BT in young compared with older patients. Moreover, our study shows some strengths; few studies have been performed on functional outcomes in young patients after BT and to the best of our knowledge, this study is the first reporting on Quadrella according to age. Although the Trifecta evaluation after radical prostatectomy has been mainly assessed at 1 and 2 years, the results presented here included 6 years analysis [12]. In addition, functional assessments were performed using validated self-administrated questionnaires (IIEF-5, IPSS) and urinary toxicity was evaluated according to widely used Crook index [10]. Lastly, the proportion of lost to follow-up patients in the long-term was fully acceptable, with a score of < 30% at 5 years and < 40% at 6 years.
Conclusions
In men aged ≤ 60 years at the time of treatment for LR-FIR PCa, BT is a first-rate option because the oncological results appear to be at least equivalent to older patients, in whom BT has demonstrated its long-term effectiveness. The good tolerance of this long-term treatment should favor this treatment in young patients, for whom the side effects of treatment are more problematic. It would be relevant to compare the results of composite BT scores in young patients with those after radical prostatectomy or EBRT.



