Introduction
Population-based neonatal screening programs aim to detect severe congenital diseases before the onset of the first symptoms. This is a prerequisite for timely initiation of therapy and for prevention of complications and/or death. In the 1960s, R. Guthrie and A. Susi (1963) initiated mass neonatal screening for phenylketonuria [1]. J.M.G. Wilson and F. Jungner (1968) defined the criteria for including a disease in the screening programs [2]. Now, thanks to the introduction of new technologies, screening programs can detect over 50 different congenital diseases. Neonatal screening panels vary considerably worldwide. Among European countries, the number of disorders included in neonatal screening programs varies significantly: from 1 to 30. Mass neonatal screening for phenylketonuria in Bulgaria was introduced in 1979, and screening for congenital hypothyroidism and congenital adrenal hyperplasia has been conducted since 1993 and 2010, respectively [3].
Inborn errors of immunity (IEI) are genetically determined disorders that are a result of the impaired function of one or more components of the immune system. According to the recent classification of the International Union of Immunological Societies (IUIS) for 2019, IEI comprise 406 disorders with nearly 430 different gene defects described [4]. Phenotypically IEI are subdivided into 10 categories [4] including severe combined immune deficiencies (SCID), which are characterized by defects in the maturation and development of T lymphocytes. Newborns with SCID have greatly increased risks for severe bacterial, viral, fungal and opportunistic infections. If not treated adequately SCID leads to a fatal outcome in the first years of life. Treatment includes hematopoietic stem cell transplantation (HSCT) or gene therapy. HSCT achieves significantly better results if it occurs in the first 3.5 months after birth and before the onset of the first SCID-related infection [5, 6]. As SCID completely meets the criteria for neonatal screening, nowadays the quantification of T-cell receptor excision circles (TRECs) in dried blood spot (DBS) samples alone or combined with kappa-deleting recombination excision circles (KRECs) is an effective strategy for timely identification of IEI in many countries worldwide [7, 8]. One of the first steps in the overall strategy for including SCID in national screening programs is to conduct a pilot study [9-14]. Data from such studies provide information on technical and logistical aspects related to the methodology used in a particular socio-economic community. Thereby optimal cut-off values, frequency of re-testing needed, frequency of false positive samples, number of clinical referrals if applicable, etc., and as best practice evaluation of the cost effectiveness are determined. In some cases these data contribute to detection of SCID due to founder mutations and give important information needed to approximate the expected SCID incidence for a given population. The aim of our pilot study was to implement the TREC/KREC testing approach in order to evaluate the compatibility and applicability of the protocol for launching a screening program for SCID in Bulgaria.
Material and methods
Newborn screening
Guthrie cards of 2,228 newborns were prospectively collected as part of the current national screening program in the National Genetics Laboratory in Sofia. The use of Guthrie cards for the purpose of this project was approved by the Local Ethic Committee. We applied two assay protocols sequentially. Initially 1,276 samples, received in December 2019, were tested for the level of TRECs, together with β-actin as a control amplicon to assess replication, using a commercial EnLite Neonatal TREC kit (Wallac Oy, Mustionkatu 6, FI-20750 Turku, Finland). In the second stage of the study we updated the protocol with simultaneous TREC and KREC quantification using the EnLite TREC-KREC kit by the same manufacturer, testing an additional 952 samples, received in April 2021. Both protocols included initial testing of the samples in singlicate and retesting in duplicate of the samples with TREC/KREC values below a certain cut-off value. For assessment of TREC and β-actin (ACTB) cut-off values in the first stage, we used the manufacturer’s recommended limits. Results with TREC ≥ 36/µl were accepted as negative and those with TREC ≤ 36/µl as positive. ACTB copy of ≥ 56 copies/µl indicated satisfactory amplification of the internal control. There was no recommended cut-off value for KREC in the second stage of the study, so this value was determined based on the data obtained from the tested samples. The intra-percentile approach was used, with the first percentile taken as the cut-off value for KREC (refer to results section, Table 1). Results with KREC ≥ 13 copies/µl were accepted as negative and those with KREC ≤ 13 copies/µl as positive. As β-actin was used only as an amplification control in the retest phase, the manufacturer did not recommend the cut-off value for it in the TREC/KREC kit, but instead a preset DAF (DNA amplification failure) limit below which the software flags the result.
Table 1
Descriptive statistics of the study
| Analyte | n | Percentile values* (copies/µl or CFCs) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1.00% | 2.00% | 2.50% | 3.00% | 4.00% | 5.00% | 50.00% | ||
| TREC | 2228 | 17 | 21 | 22 | 23 | 26 | 27 | 87 |
| β-actin 1** | 1276 | 8 | 10 | 11 | 11 | 13 | 15 | 127 |
| KREC | 952 | 13 | 17 | 18 | 21 | 23 | 29 | 94 |
| β-actin 2 | 952 | 7451 | 7890 | 8104 | 8281 | 8986 | 9501 | 21924 |
Demographic data including date of birth, date of sample collection, ethnicity of the parents, newborn sex, gestational age and weight at birth as well as application of medical substances affecting the immune system during pregnancy or transfusion of biological materials were recorded and were taken into consideration in the analysis of the test results (refer to Results section).
Excel 2019 and SPSS 26.0 (Microsoft Corporation, Chicago, Illinois, USA) were used for statistical analysis of the data. Categorical variables are given as percentages. Quantitative variables were tested for distribution normality (Kolmogorov-Smirnov test or Shapiro-Wilk test) and were presented as mean and standard deviation or median and interquartile range (IQR). Given the nonparametric distribution of the data, the Kruskal-Wallis test and the Mann-Whitney U test were used to compare quantitative variables between the groups. All the statistical analyses used were two-tailed. Reported differences were considered statistically significant at p < 0.05.
TREC and KREC detection and prediction capacity
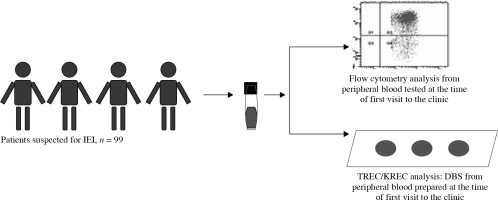
According to study design no additional blood collection or follow-up data of the newborns tested were possible or available. Data from retrospective analysis of previously collected Guthrie card samples (at birth or later) from 34 patients with confirmed IEI [15] were used to test the validity of TREC/KREC cut-off values and the scope of the assay. To assess cut-off values for TREC in adult IEI patients, blood samples from 24 healthy controls, aged 19-68 years (mean 38.6 years), were included in the study. Evaluation of TREC/KREC correspondence with lymphocyte subpopulations, defined by flow cytometry and evaluation of correlations between TREC and KREC with immune cells, were done based on the data from a total of 99 patients with suspected immune disorders (39.4% female, 60.6% male, average age 20.2 years) assessed in the Department of Clinical Immunology at the Alexandrovska University Hospital, Sofia. The main criteria for inclusion of individuals for this analysis was frequent infections. Multicolor flow cytometric immunophenotyping (FACS Canto II, FACSDiva v6.2q, Becton Dickinson) was used to identify CD3+, CD4+, CD8+ and CD19+ cells. Graphical description of this analysis is presented in Figure 1. Informed consent to use medical and immunological records for the aim of this study was obtained from participants or their parents.
Fig. 1
Study design of tested patients and samples in order to evaluate TREC and KREC prediction capacity for T- and B-cell lymphopenia
The Shapiro-Wilk test was used to assess the normality of the distribution of variables. Since the null hypothesis for normality was rejected, the Spearmen coefficient was used to estimate the strength of the correlation between the variables. The area under the curve (AUC) was represented with a 95% confidence interval, thus complementing the graphical visualization of the model. The results were considered statistically significant at a p-value < 0.05. All calculations were made by SPSS software package version 16.0.
Results
Screening results
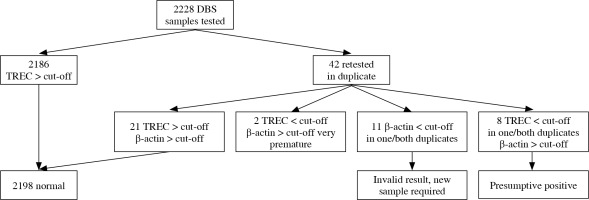
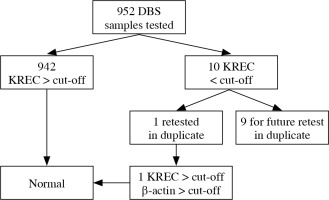
Of the 2228 samples tested, 2186 (98.11%) had TREC values above the cut-off and 42 were retested in duplicate. After retesting, normal TREC values were obtained for 21 of the samples, two of the low-score samples were in very preterm infants, 11 (0.49%) had unsatisfactory replication of the control amplicon, and 8 (0.36%) were rated as probably positive (Fig. 2). 952 samples were tested for KREC. Of them 942 (98.95%) were negative and 10 (1.05%) had KREC below the cut-off (Fig. 3). One of the positive KREC samples was retested in duplicate and gave a normal result. Due to economic and organizational issues the remaining 9 presumably positive samples will be retested at the next stage. Thereafter objective data on the percentage of samples with suspected B-cell lymphopenia will be obtained.
TREC and KREC levels in relation to demographics
The results for TREC and KREC, estimated by sex, ethnicity, birth weight, and gestational age, are summarized in Table 2. For the entire study population, the median gestational age was 39 weeks (IQR 38-40), and birth weight was 3200 g (2850-3500 g). The average weight for boys was 3250 g and for girls 3100 g. Full-term newborns comprised 87.75%, and babies with normal birth weight comprised 90.31%. Gestational age and birth weight of newborns were defined according to WHO definitions as follows: ≥ 37 weeks – full-term newborns, < 37 weeks – preterm; > 2500 grams – normal weight, ≤ 2500 grams – low birth weight [16, 17].
Table 2
Demographic data and TREC and KREC values for the population tested
For the entire study population, the medians of TREC and KREC were 87 copies/µl (54-140) and 94 copies/µl (68-133) respectively. In girls the median TREC was 91 (56-149) and in boys 83 (52-131) (p < 0.05). Premature infants and low birth weight infants (≤ 2500 g) had lower TREC values than full-term infants and normal birth weight infants (p < 0.05). No statistically significant differences between the tested groups were reported for TREC and KREC in regard to ethnicity as well as for KREC with respect to gender, gestation and weight at birth.
TREC and KREC detection and prediction capacity
Testing results of IEI patients with confirmed diagnosis
The secondary aim of the project was to apply TREC/KREC testing to patients with confirmed IEI in order to adjust test cut-off levels for both analytes as well as to assess the capacity of the method to uncover disorders other than SCID. In two patients (P1 and P2) with severe cellular and humoral immune deficiencies (T-B-phenotype) no TREC and KREC were detected at birth (Table 3). Patient 1 had hypomorphic RAG1 mutation and was clinically stable at the age of 7 years when she was tested again for TREC and KREC but in peripheral blood. The results showed low levels of TREC and KREC, which corresponded to the low absolute number of her T- and B cells (CD3+ 522 cells/µl, CD19+ 6 cells/µl) at that time. Patient 3 had less profound deficiency than SCID; 24 copies/µl TREC and 13 copies/µl KREC were found in DBS at birth. From the group of combined immunodeficiencies (CID) with associated or syndromic features we have tested 7 patients. Patients P4-P7 have been diagnosed with deletion in chromosome 22. Only in one (P7) out of four patients were TRECs below the cut-off value. Both patients with immune deficiency due to DNA repair defects – ataxia telangiectasia (AT) (P10) and Nijmegen breakage syndrome (NBS) (P9) – were tested positive with low TREC and KREC values, more pronounced in AT. The single patient with hyper IgE syndrome had normal TREC and KREC results. From the group of predominantly antibody deficiencies practically absent KREC at birth was found in the patient with X-linked agammaglobulinemia (XLA) (P11). Patients with common variable immune deficiency (CVID) consisted of 14 predominantly adult patients with average age 45.57 ±13.4 years. Interestingly, all of them presented with abnormal TREC and/or KREC; among them, 11 pa-tients (78.5%) had TREC numbers below 36 copies/µl, 12 patients (85.7%) had low KREC values and 50% had low values of both indicators in PB at the given age (Ta-ble 3). Mean TREC and KREC values for the CVID group were 17.2 copies/µl and 22.93 copies/µl. In comparison, mean TREC and KREC values in healthy adults were 108.9 and 73.6 copies/µl respectively (preliminary data of a small cohort with mean age 38.6 years). The child with transient hypogammaglobulinemia of infancy (P26) had normal TREC and KREC values. Data from the patients referred to classification groups of IEI other than those already described herein revealed slightly decreased levels of TRECs in a patient with STING-associated vasculopathy (P27) and in a patient with mannose-binding lectin deficiency (MBL).
Table 3
Results of TREC and KREC testing of patients with confirmed IEI
* According to 2019 International Union of Immunological Societies Phenotypical Classification [4]
DBS (dried blood spots) at birth – blood for the test was taken after the forty-eighth hour (second day) and before the ninety-sixth hour (fourth day) for newborns with weight at birth ≥ 2,000 g and on the fourth day of the child’s life, at the end of the second week or in case of earlier discharge – on the day of discharge for newborns with weight at birth ≤ 2,000 g; DBS at birth were tested retrospectively. DBS-PB – dried blood spots prepared from peripheral blood taken at the time of testing and corresponding to the patient’s age individually presented in the table; SCID – severe combined immune deficiency; AR – autosomal recessive; MHC – major histocompatibility complex; CVID – common variable immunodeficiency; FMF – familial Mediterranean fever; PFAPA – periodic fever, aphthous stomatitis, pharyngitis, adenitis; CGD – chronic granulomatous disease; G6PD – glucose-6-phosphate dehydrogenase; NT – not tested.
TREC and KREC testing in patients with suspected immune disorders and lymphocyte subpopulations
The initial analysis of our data included the assessment of 4 paired relationships between TREC levels with CD3+, CD4+, CD8+ and KREC with CD19+ cells. They were presented as the proportion of patients with normal levels for one of the paired variables, among patients with normal levels of the other immunological marker. We also assessed the correlations between TREC/KREC and lymphocyte subpopulations. Further, we evaluated the ability of TREC and KREC to predict an abnormality in the number of a given subpopulation of lymphocytes.
ROC analysis was used to test the predictability of TREC and KREC, which provides sensitivity assessment at fixed specificity as well as AUC. The cut-off values used for abnormality of TREC and KREC were the same as those used for the interpretation of newborn screening results. Cell populations were defined as abnormal when a percentage or absolute number are outside the laboratory established age-specific and population-specific reference intervals.
Comparative analysis of flow cytometric parameters with TREC and KREC values
Our results revealed that 50 of 69 (72.5%) patients with normal KREC levels had normal absolute CD19+ cell counts, while 50 of 58 (86.2%) individuals with normal CD19+ counts had normal KREC values. In patients with normal KREC levels, 45 of 69 (65.2%) cases of CD8+ were found within the age reference range. In patients with normal TREC levels, 44 of 66 (66.7%) had a normal CD3+ cell count, 39 of 66 (59.1%) had a normal CD4+ cell count, and 45 of 66 (68.2%) had a normal CD8+ cell count. Individuals with normal absolute CD3+ levels had normal TREC levels in 44 out of 51 cases (86.3%). Of patients with a normal CD4+ cell count, 39/44 (88.6%) had normal levels of T cells. In the case of individuals with a normal CD8+ count, normal TREC levels were found in 45/54 (83.3%).
Correlation between TREC/KREC values and lymphocyte subpopulations
We observed a moderate correlation between TREC levels and absolute values of CD4+ (r = 0.634, p < 0.01), TREC and CD3+ (r = 0.536, p < 0.01) lymphocytes, TREC and CD19+ (r = 0.519, p < 0.01) and KREC levels with the number of CD19+ positive cells (r = 0.497, p < 0.01) (Table 4). A moderate to low correlation was found between TREC with absolute values of CD8+ lymphocytes (r = 0.350, p < 0.01) and KREC with CD3+ lymphocytes (r = 0.311, p < 0.01), and with CD4+ T cells (r = 0.312, p < 0.01), as well as with the percentage of CD19+ cells (r = 0.416, p < 0.01). There was also a statistically significant correlation between TREC levels and the percentage of CD19+ cells and CD4 lymphocytes (respectively r = 0.310, p < 0.01 and r = 0.223, p < 0.05).
Ability of TREC and KREC values to predict abnormal levels of lymphocyte subsets
We evaluated the ability of TREC/KREC to predict different lymphocyte subsets using area under the curve (AUC) analysis. The AUC provides information on how well the model is able to distinguish between positive and negative results. The constructed ROC curves visualize the extent to which the model is reliable in prognosing the presence of abnormal levels of lymphocyte subpopulations. The results for each cell population studied are shown in Supplemental material.
TREC demonstrated an AUC of 0.805 (95% CI: 0.71-0.90) for CD3 with respect to the absolute cell count and an AUC of 0.595 (95% CI: 0.48-0.72) for the percentage of T cells. The TREC ROC analysis for CD4 revealed an AUC of 0.847 (95% CI: 0.77-0.93) for the absolute number and an AUC of 0.619 (95% CI: 0.50-0.73) for the percentage of CD4+ cells. The evaluation of TREC for CD8+ showed an AUC of 0.731 (95% CI: 0.62-0.85) for the absolute number and 0.460 (95% CI: 0.33-0.59) for the percentage of suppressor-cytotoxic T cells. Subsequent TREC analysis found an AUC of 0.756 (95% CI: 0.66-0.86) with respect to the absolute CD19+ values, and an AUC of 0.636 (95% CI: 0.51-0.76) for the percentage of B lymphocytes. The analysis of the ability of KREC levels to predict abnormal levels of lymphocyte populations showed an AUC of 0.772 (95% CI: 0.65-0.89) for the absolute CD19 count and 0.731 (95% CI: 0.59-0.87) for the percentage of B cells.
Discussion
Infants with SCID and other forms of IEI are susceptible to life-threatening infections of different origins, as well as secondary infections induced by live vaccines [18]. Early diagnosis was made possible by testing T- and/or B-cell lymphopenia with newborn screening (NBS) procedures, thereby significantly improving the life of affected children. Nowadays, more and more countries worldwide have already introduced or are considering the introduction of population-based NBS for IEI. The combined TREC/KREC approach can significantly advance early diagnosis of agammaglobulinemia, Nijmegen breakage syndrome, ataxia-telangiectasia or DiGeorge (DGS) syndrome, as well as late-onset ADA SCID [11, 19-21]. Diagnostic protocols and TREC cut-off values vary considerably among different countries, with TREC cut-offs ranging from 7 to 252 TRECs/µl and different retest strategies, including equal or different cut-offs for the initial test and for retesting of the samples [22, 23]. In the current study we decided to use the manufacturer’s recommended limit for TREC of 36 copies/µl for both initially tested and retested samples in order not to miss any newborns with combined immune deficiencies less profound than severe. Results of the retrospective testing of NBS cards of known IEI patients supported this strategy. With the cut-off value of 36 copies/µl for TREC, the retest rate was 1.88%. This corresponded with retest rates reported in previous studies when similar cut-off thresholds have been used [10, 12, 24-26]. In most of these studies retest rates have been assessed against different cut-off values, and in order to reduce retests, lower cut-offs are recommended. Nevertheless, according to the results of retrospective testing, a cut-off limit of 36 copies/µl might be suitable for detection of IEI in the Bulgarian population. Despite the different methods used (end-point PCR using a commercial EnLite Neonatal TREC-KREC kit or RT-PCR by means of home-made methods or commercial kits, e.g. SPOT-it), the obtained values of TREC were found to be similar to TRECs reported in other publications. Median TREC for the entire studied population was 87 copies/µl, compared to 86 copies/µl in Andalusia and the Polish-German transborder area [27, 28], 93 copies/µl in Seville [11] and 96 copies/µl in the Dutch population [25]. Associations of TREC levels with the demographic variables gestational age, birth weight and gender were also found in previous studies in Spain and Israel [11, 14, 27]. The median TREC value for preterm babies was 76 copies/µl compared to 88 copies/µl in full-term babies (p < 0.05). Reduced thymic output due to immature thymus development could be one possible reason for the lower TREC values in premature and low birth weight babies. Atkins et al. estimated that the TREC values increase at a steady rate of 9.8% per week as gestational age increases [29]. As according to our study design obtaining a second heel prick from the preterms with low TREC values was not possible, a lower cut-off value can reduce the higher retest rate in this sample [30]. To our knowledge, there is no explanation in the literature for the difference in TREC levels with regard to gender. Further studies may find out the possible reasons for this difference.
The observed KREC levels (median 94 for the entire studied population) correspond with the median of 100 copies/µl in the Swedish population [9] and are considerably higher than KREC medians reported in Spain, the Polish-German transborder area and Iran [11, 27, 28, 31]. These differences are probably due to the different methods used for KREC quantification – RT-PCR vs. the commercial EnLite Neonatal TREC-KREC kit. No statistically significant differences were reported for KREC in regard to gestational age and weight at birth, unlike the findings in previous studies [27, 28]. Since we have tested for KREC a relatively small sample number, no conclusions for the whole population should be made. Data from Sweden show that a relatively large proportion of false-positive results are due to KREC, but the level of re-requested samples is nevertheless considered acceptable [32]. The cost of TREC testing alone is in some cases comparable to that of using the combined method, which further justifies the use of this approach. To assess whether the cost and the retest rate for KREC testing are acceptable for the conditions in Bulgaria, we need to collect additional data.
End-point PCR has some limitations compared to real- time PCR in terms of accuracy, execution time and hand labor. PerkinElmer are introducing a real-time PCR based assay in place of the end-point PCR based EnLite assay. Taking into account the fact that the design of this assay and system enables automation and the advantages of real-time PCR over end-point PCR, it is worth considering switching to a commercial RT-PCR assay after appropriate validation, if newborn SCID screening is approved for implementation in Bulgaria.
Our results from retrospectively tested patients with confirmed IEI revealed that the TREC/KREC assay had 100% sensitivity to identify SCID cases (no detectable TREC and KREC) when the DBS were obtained at birth. These results gave us additional confidence that in the conditions of a future screening program in Bulgaria the assay protocol would fully justify the capacity for which it has been constructed and validated [23]. Opposite to the observation that TREC screening would not be able to identify SCID in which a genetic defect lies downstream of T-cell receptor rearrangement, including IEI such as ZAP70, MHC class II (major histocompatibility complex class II), and ADA (delayed onset disease) deficiencies [33-35], we demonstrated that the assay was capable of identifying a single patient with MHC class II deficiency. The values of 24 TREC copies/µl and 13 KREC copies/µl detected in DBS of this patient showed that at this stage in order to identify newborns with combined immune deficiencies generally less profound then severe, it seems reasonable to adhere to the TREC cut-off values recommended by the manufacturer, and KREC cut-off values calculated as described above. Retrospective evaluation of other similar cases would help to clarify the scope of the assay to detect immune deficiencies other than SCID.
According to previous reports TREC or TREC/KREC testing is usually capable of identification of selected CID other than SCID [31, 36]. This potential was supported by our results in terms of AT, NBS and X-linked agammaglobulinemia. However, DGS syndrome, although included in the list of IEI recognizable by NBS programs, was missed in one out of two newborns tested at birth. How- ever, according to some studies nearly 1 in 27,000 newborns underwent immunologic evaluation due to low TREC numbers and were found to have 22q11 deletion [37]. Barry et al. [38] revealed retrospectively that only 11 out of 1,350 patients with DGS syndrome tested positive at birth screening. These discrepancies could be explained mainly by the extreme immunological and clinical heterogeneity as well as incomplete penetrance of the partial form of the disease [39]. Surprisingly, the results of TREC/KREC testing in patients with CVID showed that in all of them there was a deviation in the values of at least one of the two indicators. These patients were genetically evaluated and CID or other forms of IEI were thus excluded. Kamae et al. found that the subgroup of patients with CVID had abnormalities in TREC/KREC levels so the authors proposed a classification algorithm according to data on excision circles. Furthermore, they summarized that TREC/KREC testing in this cohort could serve as an independent marker to determine the patient’s immunologic status and clinical outcome as well as to distinguish between CID and CVID [40]. Another study also revealed that low TREC and KREC values reflected low naïve T and B lymphocytes in selected CVID patients with the potential to develop CID phenotype [41]. However, according to Korsunskiy et al. TREC and/or KREC failed to demonstrate good CVID diagnostic ability [42].
In the context of changes in TREC and KREC values that we found in patients with CVID, it is worth conducting future retrospective testing of DBS at birth in order to evaluate the possibility that newborns with established abnormalities in TREC/KREC during NBS will manifest the disease later in life. As TREC values decrease with age, additional testing of a larger cohort of healthy adults could help to set cut-off limits for the different age groups in the Bulgarian population.
In the present study, we analyzed the capacity of TREC and KREC to predict abnormalities in the number of general immune cell populations, and correlations between TREC/KREC levels and individual lymphocyte subtypes. The results showed the strongest significant correlations between TREC and the absolute numbers of CD4+ cells (r = 0.634, p < 0.01), and total T cells (r = 0.536, p < 0.01), pointing out that low TREC levels reflect a low number of CD3+ cells, particularly their helper-inducer subsets. This finding was consistent with some other studies [43, 44], as the correlations we have obtained are even more pronounced than previously reported [44]. A significant moderate correlation was found between TREC and CD19+ cells (r = 0.519, p < 0.01) and KREC and CD19+ cells (r = 0.497, p < 0.01), which was more pronounced than reported by Korsunskiy et al. The results justified the assumption that the levels of both TREC and KREC significantly reflect the reduced absolute values of B lymphocytes. Comparative analysis revealed that the percentage of patients with normal values of CD3+, CD4+, and CD8+ cells having normal values of TREC was above 83% (83.3-88.6). Eighty-two percent of patients with B cells within age-related reference ranges had KREC above the accepted cut-off level. The results presented so far are in logical accordance with the obtained models for the ability of TREC and KREC to predict changes in immune cells. The best predictive capacity demonstrated TREC relative to the absolute numbers of CD4+ T cells, followed by total T cells, CD8+ and B cells. However, relative to the percentages of the same cell populations, the predictive capacity of TREC seems to be limited. The ability of KREC to predict the absolute numbers of B lymphocytes was found to be promising, as previously reported [43].
The current first-of-its-kind pilot screening study is the basis for future actions to include TREC/KREC testing in the national screening program. Thanks to this scientific initiative, numerous meetings were held with representatives of the Ministry of Health, the National Health Insurance Fund, the Bulgarian Association of Clinical Immunology and the Bulgarian Pediatric Association, and non-governmental organizations. The project has been submitted for consideration by expert committees of the Ministry of Health. An evaluation of the benefits of the introduction of TREC/KREC screening program in accordance with the costs, as well as the development of a logistical organization, is pending.
The main limitation of this study is related to the small number of the screened samples. A study with larger sample size as a second step could help to establish population and gestation age based cut-off values and to reduce the retest rate. A very limited number of SCID cases were included in the validation group of CID patients, because, according to the Bulgarian national register of patients with primary immune deficiencies, only 5 patients were registered with severe combined immune deficiency and 6 patients with combined immune deficiency, generally less severe that SCID. Nevertheless, accordance between our results and those reported in larger cohort studies supports their validity and demonstrates the effectiveness of the combined TREC/KREC assay for the purposes of newborn screening at the national level.
Conclusions
This study demonstrates that newborn screening for SCID, XLA and other forms of IEI at birth using the TREC/KREC assay has high diagnostic accuracy and is effective and reliable. TREC levels showed associations with gestational age, birth weight and gender of the newborns. There were significant correlations between TREC and the absolute numbers of CD4+ cells and total T cells, pointing out that low TREC levels reflect a low number of CD3+ cells and particularly their helper-inducer subsets. A significant moderate correlation was found between TREC and CD19+ cells and KREC and CD19+ cells.