Summary
This study may elucidate the mechanism responsible for periprocedural myocardial injury/infarction in the presence of a low level of insulin-like growth factor-1.
Introduction
Periprocedural myocardial necrosis, which can range from a low-level elevation of cardiac biomarkers to a large myocardial infarction (MI), is a common complication after percutaneous coronary intervention (PCI). While advances in interventional technology and techniques have translated into a reduction in procedure-related morbidity and mortality, the incidence of procedural myocardial injury/infarction has not changed significantly [1]. However, MI associated with percutaneous coronary intervention (type 4a MI) is defined by a post-procedural rise of troponin values to five times the 99th percentile upper rate limit (URL) in patients with normal baseline values, or a rise in troponin values by 20% if the baseline values are elevated but are stable or falling. The Universal Definition further mandates that the post-procedural elevation of troponin should be accompanied by clinical, electrocardiographic (ECG), echocardiographic or angiographic evidence of myocardial ischaemia.
Insulin-like growth factor-1 (IGF-1) is important for the plaque stability in atherosclerosis due to its positive effects on vascular smooth muscle cells (VSMC) [2]. Since IGF-1 plays an important role in VSMC apoptosis and migration, low levels may play an important role in acute coronary syndrome by promoting plaque instability [3]. Moreover, it was shown that IGF-I is involved in the repair of the intima in injured arteries. Smooth muscle cell accumulation is important for the neointimal proliferation after angioplasty. Hence, IGF-1 can be effective for neointimal proliferation after angioplasty [4].
Optical coherence tomography (OCT) is an intravascular imaging method that demonstrates the detailed visualization of plaque components and morphology and is used for the optimization of procedural outcome [5]. OCT allows for accurate determination of fibrous cap thickness, thereby enabling in vivo identification of the presence of thin-cap fibroatheroma (TCFA) [6]. The presence of a thin fibrous cap or increased lipid core on intravascular imaging is a predictor of a periprocedural MI. OCT-based fibrous cap thickness is the most important predictor of periprocedural MI [7].
Aim
In the present study, we hypothesized that IGF-1 levels may play a protective role in myocardial injury after coronary stent placement and aimed to investigate the relationship between IGF-1 levels and plaque characteristics assessed by OCT.
Material and methods
Study population
Between May 2015 and December 2015 we prospectively enrolled 74 patients with stable angina pectoris in whom single de novo coronary artery stenosis was present. We performed PCI in the case of lesions described as significant stenosis. Significant stenosis was defined as angiographic > 50% diameter stenosis in patients with ischemia that was detected during myocardial perfusion scintigraphy or the treadmill exercise test. Stenosis > 50% in the absence of ischemia testing was defined as significant if the fractional flow reserve (FFR) value of the stenosis was < 0.80. Patients with acute coronary syndrome or elevated high-sensitivity troponin T (TnT) level at admission were excluded. Also, previous coronary artery bypass grafting, patients with bifurcation lesions and previous myocardial infarction, lesions involving the left main stem and chronic total occlusions were excluded from the study to take optimal OCT measures. Patients with an estimated glomerular filtration rate below 60 ml/min (by the Cockcroft and Gault formula) and use of insulin were excluded as well. All type 2 diabetic patients were on metformin treatment. All patients provided written informed consent and the study was approved by the institutional review board.
Clinical and laboratory data
All clinical features of the patients were collected at admission. Creatinine, glycated hemoglobin (HbA1c) and lipid profile were determined by standard methods. The assay used in our center measures cardiac troponin T (cTnT) with high sensitivity using the immuno-analysis ECL-sandwich by Roche. The lower detection limit was 0.01 ng/ml (99th percentile of reference control group). TnT was analyzed at admission, before PCI and at 6, 12, 24 and 48 h after PCI. For IGF-1, at the baseline examination, 3 ml of serum from each participant was obtained from subjects in a supine, nonfasting state in the afternoon and stored at –80°C. Serum IGF-1 was measured by radioimmunoassay after acid–ethanol extraction in 3 batches16; the intra-assay coefficient of variation was < 4%. Insulin-like growth factor binding protein levels were not measured. The assay used in our center measures TnT with high sensitivity using the immuno-analysis (ECL-sandwich by Roche). According to the fourth universal definition of myocardial infarction [8]; cardiac procedural myocardial injury was defined by increases of cTn values (> 99th percentile URL) in patients with normal baseline values (≤ 99th percentile URL) or a rise of cTn values > 20% of the baseline value when it is above the 99th percentile URL but it is stable or falling; and coronary intervention-related MI was defined by an elevation of cTnT values more than five times the 99th percentile URL in patients with normal baseline values. In patients with elevated pre-procedure cTn in whom the cTnT level is stable (≤ 20% variation) or falling, the post-procedure cTnT must rise by > 20%. However, the absolute post-procedural value must still be at least five times the 99th percentile URL. In addition, one of the following elements is required: new ischaemic ECG changes; development of new pathological Q waves; imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischaemic etiology; angiographic findings consistent with a procedural flow-limiting complication such as coronary dissection, occlusion of a major epicardial artery or a side branch occlusion/thrombus, disruption of collateral flow, or distal embolization.
PCI procedure
PCI was performed according to standard methods, and images were stored digitally. 300 mg aspirin, 600 mg loading and 75 mg daily clopidogrel doses were administered to all study patients. Glycoprotein IIb/IIIa inhibitors were used in 1 patient who developed no-reflow. All patients received weight-adjusted intravenous unfractionated heparin. To all patients before and after PCI, OCT was applied in the culprit vessel only, specifically for this study. To obtain images of basal lesions, a C7XR LightLab Dragonfly OCT catheter (St. Jude Medical, Little Canada, MN, USA) with an automatic motorized pullback system (20 mm/s) was used after administering 200 μg of intracoronary nitroglycerine. The catheter was advanced distally to the lesion over a conventional 0.014-inch guidewire and images were obtained nonocclusively by motorized pullback at 20 mm/s during 4 ml/s flushing with a total of 14–16 ml of contrast media, Visipaque (GE Health Care, Cork, Ireland) or Iomeron (Ilsung Corp., Seoul, Korea). Prior to predilatation, stent diameter and length were determined in accordance with OCT images and at the discretion of the primary operator. All patients received a Xience pro (Abbott Vascular) stent implanted with 14 atm pressure. OCT images were acquired immediately using the same catheter following stent implantation. Afterwards, either termination of the procedure, the requirement of postdilatation with a noncompliant balloon, or additional stent implantation was decided in accordance with the last OCT images, angiogram, and at the discretion of the primary operator. Following this, once again OCT acquisition was taken for stent optimization and index parameters in cases of additional stent implantation and/or noncompliant balloon dilation. In the presence of stent edge dissection in only OCT images without angiographic evidence, an additional stent was not implanted. No-reflow was defined as thrombolysis in myocardial infarction (TIMI) < 3 distal flow at the index procedure. Hemodynamic complications during the procedure were defined as hypotension and dysrhythmia, and angiographic complications were defined as any coronary dissection, no-reflow or vessel rupture.
OCT quantitative analysis
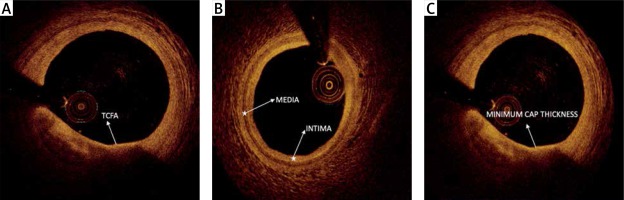
Optical coherence tomography quantitative analysis was performed by an independent observer using the C7-XR FDOCT system (St. Jude Medical). The system was calibrated for z-offset and images were analyzed frame by frame. Lesion lengths and stent lengths were measured in longitudinal images by the device in a St. Jude Workstation. On each OCT frame within the stent, all struts were counted and each strut was evaluated for stent apposition. Malapposition was defined in cases where the distance from the surface of the strut to the lumen contour was longer than the implanted stent strut thickness. A consensus of 2 independent observers for each frame was obtained. Cross-sectional OCT images were analyzed frame by frame. Cholesterol crystal was defined as a thin linear region of high signal intensity within a lipid plaque [9]. Stent edge dissection was defined as disruption of the luminal vessel surface in the edge segments within 5 mm proximal and distal to the stent while no struts are visible [10]. Lipid plaque was defined as a signal-poor region with diffuse borders, calcified plaque as a well-delineated, signal-poor region with sharp borders, and fibrous plaque as a homogeneous signal-rich region [11]. Plaque type at a patient level was defined according to the lesion which is causing only critical stenosis. Plaque length was measured by ruler in longitudinal images. The plaque arc of the most critical section of each lesion was measured manually by an electronic goniometer in cross sectional images. Lipid volume index was defined as the mean lipid arc multiplied by lipid length [12]. Reference intimal thickness, reference medial thickness, and plaque fibrous cap thickness were measured by ruler in cross-sectional images (Figure 1). Minimal lumen area (MLA) was defined as the smallest lumen area along the length of the target lesion and reference lumen area (RLA) was defined as the site with the largest lumen either nearest proximal or distal to the stenosis within the same segment. Percent luminal area stenosis was defined as the relative decrease in the luminal area of the target lesion when compared with the reference lumen in the same vessel segment. These measurements were calculated by the device automatically. Minimal lumen diameter (MLD) was defined as the smallest lumen diameter along the length of the target lesion and reference lumen diameter (RLD) was defined as the site with the largest lumen diameter either nearest proximal or distal to the stenosis within the same segment [9]. Post-stent luminal mass was defined as the presence of thrombus or plaque materials in the lumen after stent implantation in OCT images [13]. Spotty calcification was defined as the presence of lesions < 4 mm in length and containing an arc of calcification < 90° in OCT images [14]. Thrombus was defined as an irregular mass with minimum diameter at least 250 μm adherent to the vessel wall or floating within the lumen in OCT images. Microvessels within the intima in plaque were defined as signal-poor voids that are sharply delineated and usually can be followed in multiple contiguous frames. Plaque rupture was defined as a fibrous cap discontinuity with cavity formation. Macrophages were defined as signal-rich, distinct, or confluent punctate regions that exceed the intensity of background speckle noise. Calcified nodules in OCT (a calcific nodule is defined as a single or multiple regions of calcium (defined previously) were defined as masses protruding into the lumen that frequently form sharp and jutting angles. An OCT thin-capped fibroatheroma (OCT-TCFA) was defined as an IVOCT-delineated necrotic core with an overlying fibrous cap where the minimum thickness of the fibrous cap is less than 65 mm [9].
Statistical analysis
The patients were divided into two groups according to presence of myocardial injury or type 4a myocardial infarction following percutaneous coronary intervention: the first group (myocardial injury or type 4a myocardial infarction, n = 25) and the second group (no myocardial injury or type 4a myocardial infarction, n = 49). Continuous and categorical variables were expressed as mean ± standard deviation and percentages, respectively. Group means for continuous variables were compared using the Mann-Whitney U test and independent sample t-test. Categorical variables were compared using the χ2 test or Fisher’s exact test, as appropriate. In order to perform the univariate comparison between IGF-1 level with reference intimal thickness, reference medial thickness and plaque fibrous cap thickness, Spearman and Pearson correlation analysis was applied. To determine the independent predictors of myocardial injury or type 4a infarction, univariate and multivariate logistic regression analysis models were applied. For the multivariate logistic regression analysis model, apart from IGF-1 level, diabetes mellitus, presence of intra-plaque macrophage and cholesterol crystal, HbA1c level, presence of stent edge dissection and post-PCI luminal mass, and antiplatelet therapy were included. Odds ratio (OR) and 95% confidence intervals (95% CI) were calculated. At this scope, all variables showing a significance value < 0.05 in correlation analysis were included in the model. In all statistical analysis, two-tailed p-values of < 0.05 were considered to indicate statistical significance. ROC curve analysis were used to calculate the sensitivity and the specificity of IGF-1 for periprocedural myocardial injury and type 4a myocardial infarction. All statistical analyses were made using SPSS 11.5 (SPSS Inc, Chicago, IL, USA). The effect size (Cohen’s d) and power value (1 – β) for IGF-1, compared between patients with and without periprocedural myocardial injury/type 4a myocardial infarction, were calculated using the G Power software (version 3.1.9.2). The α level used for this analysis was < 0.05. The effect size and power value were 1.23 and 0.99 for IGF-1.
Results
We consecutively enrolled 74 patients with single de novo native coronary lesion who were treated with drug-eluting stent implantation. All patients underwent FD-OCT-guided PCI and were analyzed. A total of 25 (33.7%) patients had periprocedural myocardial injury or type 4a myocardial infarction and 49 (66.2%) patients had no events. Of 25 patients, 7 had periprocedural myocardial injury and 18 had type 4a myocardial infarction. Basal characteristics of both groups are described in Table I. Presence of type 2 diabetes mellitus (39% vs. 84%, p < 0.001) was more likely in the periprocedural myocardial injury or type 4a infarction group. HbA1c levels (5.88 ±1.10% vs. 6.90 ±1.63%, p = 0.006) and oral antidiabetic use (32.7% vs. 68%, p = 0.004) were higher in the periprocedural myocardial injury or type 4a infarction group as well. IGF-1 level (180.5 ±55.6 ng/ml vs. 128.2 ±23.6 ng/ml, p < 0.001), reference intimal (169.2 ±60.7 mm vs. 65.3 ±12.7 mm, p < 0.001), medial thickness (118 ±21.9 mm vs. 78.5 ±9.7 mm, p < 0.001) and fibrous cap thickness (163.9 ±60.2 mm vs. 59.1 ±14.2 mm, p < 0.001) were lower in the periprocedural myocardial injury and type 4a infarction group. Presence of intra-plaque macrophage (2% vs. 32%, p < 0.001) and cholesterol crystal (4.1% vs. 44%, p < 0.001) was more likely in the periprocedural myocardial injury or type 4a infarction group. All of the patients had TCFA developed periprocedural myocardial injury or type 4a infarction (n = 18). Presence of stent edge dissection (2% vs. 28%, p = 0.001) and post-PCI luminal mass (2% vs. 40%, p < 0.001) was more likely in the periprocedural myocardial injury or infarction group. Additional stent implantation was required for only 4 patients in the periprocedural myocardial injury or type 4a infarction group. There was no side branch coronary occlusion after the index PCI procedure in either group. Procedural features and OCT parameters are shown in Table II.
Table I
Baseline clinical and laboratory parameters
Table II
Procedural features and OCT parameters. Described as n and (%)
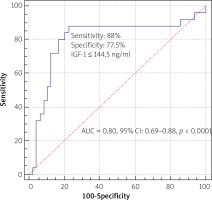
IGF-1 level and reference intimal thickness, medial thickness, and fibrous cap thickness in OCT had strong correlations (r = 0.88, 0.80 and 0.88 respectively, p < 0.001) (Table III). The level of IGF-1 was an independent predictor of periprocedural myocardial injury or type 4a MI in univariate (OR = 0.929, 95% CI: 0.895–0.964, p < 0.001) and multivariate regression analysis (OR = 0.757, 95% CI: 0.575–0.998, p = 0.04). Based on ROC analysis, the best cut-off value of IGF-1 for predicting periprocedural myocardial injury or type 4a myocardial infarction was 144.5 ng/ml, with a maximum sensitivity of 88% and specificity of 77.6% (AUC = 0.80, 95% CI 0.69–0.88, p < 0.0001) (Figure 2).
Discussion
The present study revealed that the plasma IGF-1 levels were significantly lower in the periprocedural myocardial injury or infarction group. Additionally, there was a strong correlation between IGF-1 levels and plaque fibrous cap thickness. To the best of our knowledge, this is the first study to describe the role of IGF-1 in plaque characteristics by assessing OCT as an intravascular imaging method. The cut-off value of IGF-1 above 145 ng/ml predicts periprocedural myocardial injury/infarction with 88% sensitivity and 77.6% specificity.
Myocardial damage after PCI defined as cardiac troponin elevation has been reported as a procedural complication. There are numerous methods such as OCT, intravascular ultrasound, and near infrared spectroscopy that are able to recognize lipid-rich plaques. However, the fibrous cap thickness according to OCT showed the highest probability to predict periprocedural MI. Additionally, OCT-verified TFCA was found to be an independent predictor for periprocedural MI as well as post-stent implantation such as subsequent in-stent neoatherosclerosis [15, 16]. Similarly, previous OCT studies to predict periprocedural MI have found the cap thickness and lipid arc to be predictors of periprocedural MI [17].
IGF-1 has been associated with many physiological actions, i.e. tissue growth and development, proliferation, pro-survival/anti-ageing, lipid metabolism, anti-inflammatory, antioxidant, neuro- and hepato-protective properties. Therefore, regardless of the presence of diabetes mellitus, a high level of IGF-1 leads to plaque stability. Sirbu et al. [18] showed that low IGF-1 was associated with higher abnormality of the carotid intima media. Plaque ruptures are more likely to occur in lesions with thin fibrous caps, high concentrations of lipid-filled macrophages, and those with large necrotic cores. Apoptosis and necrosis play a very important role in this condition by forming the necrotic core. Additionally, apoptosis of smooth muscle and macrophages makes lesion regression likely unfavorable. For this reason, IGF-1 deficiency has a central role in this whole process, as it is one of the most potent antiapoptotic, antioxidant and cytoprotective factors known today. Moreover, IGF-1 is also known to have vasodilator actions [19].
IGF-1 regulates cell proliferation, apoptosis, migration and differentiation, so it is a potent mitogen factor for vascular smooth cells to stimulate migration and antiapoptotic effects of VSMC [20]. Thus, the reduction of levels leads to plaque instability. An experimental study showed that IGF-1 in atherosclerotic plaques may have a role in preventing plaque instability by modulating smooth muscle cell turnover and phenotype [21]. In furtherance of this finding, the PRIME study, which evaluated the relationship between IGF-1 and occurrence of acute coronary syndromes, showed that high levels of IGF-1 appear as a negative, independent predictor of occurrence of acute coronary syndromes at 5 years [22]. Thus, according to this study, IGF-1 might have a role in plaque denudation and destabilization.
In our study, plaque fibrous cap thickness was lower in the periprocedural myocardial injury/infarction group and all patients with TCFA had periprocedural myocardial injury/infarction. Additionally, IGF-1 levels were found to be lower in these patients.
Although this study for the first time examined the relationship between IGF-1 and periprocedural myocardial injury/infarction following PCI, it also has limitations. It was a single-centered study and the number of patients was small. We only measured free IGF-1 and measurements of IGF-binding proteins (IGF-BP) were not available. Future studies that include not only IGF-1 levels, but also circulating IGF-BPs, will allow a better understanding of the mechanisms responsible for the associations observed in the present study.
Conclusions
The results from this study indicate that low IGF-1 levels are associated with plaque instability assessed by OCT. There was a strong correlation between IGF-1 level and plaque fibrous cap thickness in OCT. Patients with high IGF-1 levels had a thicker plaque fibrous cap, i.e. more stable plaque. Therefore, IGF-1 levels may play a protective role in the myocardial injury after coronary stent placement. Low IGF-1 levels may identify patients who are at increased risk for periprocedural myocardial injury/infarction. This study may elucidate the mechanism responsible for periprocedural myocardial injury/infarction. In the presence of a low level of IGF-1 prior to elective PCI, more potent antiplatelet therapy (prasugrel, ticagrelor, glycoprotein IIb/IIIa inhibitors) might be of value in preventing periprocedural MI. Further experimental and prospective clinical studies are needed to demonstrate the link between the plaque characteristics and IGF-1.