Introduction
Interleukin 25 (IL-25) belongs to the IL-17 cytokine family, which includes IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F. Stimulated by IL-1β and IL-23, IL-17A is produced by CD4+ and CD8+ T cells, γδ T cells, and various innate immune cell populations, stimulating various antimicrobial peptides, chemokines, and proinflammatory and proliferative cytokines [1]. However, sharing only 16% homology with IL-17A [2], IL-25 and IL-17A are at quite opposite poles. IL-25 was first identified by Fort et al. as a Th2-derived cytokine in various mouse cell lines and tissues, with high levels of gene expression in Th2-polarized cells and the gastrointestinal tract [3]. Apart from various immune cells, epithelial cells are also significant sources of IL-25 [2]. Therefore, IL-25 has been suggested as a “barrier surface” cytokine, released in response to external damage to barrier epithelial cells [4]. The function of IL-25 has been investigated in the physiology and pathology of various organs [5-8]. IL-25 induces overproduction of IL-4, IL-5, and IL-13 and leads to Th2-skewed inflammation [9]. Apart from promoting type 2 responses, studies have proved that IL-25 can suppress Th1 and Th17 responses in different environments including the intestine [10-12]. Apart from activity in inflammation and allergy reactions, IL-25 also may be involved in tumor- igenesis in ways to be explored yet [13, 14].
The role of IL-25 in type 2 immunity has been studied over recent years and its involvement in different diseases of multiple organs has attracted attention. The function of IL-25 in intestinal diseases, such as intestinal helminth infection, inflammatory bowel diseases, and food allergies, has been studied yet remains obscure.
Sources of interleukin 25
Interleukin 25 is widely distributed in multiple tissues and systems such as kidney, bone marrow, alveoli, the cen-tral nervous system, placenta and bronchial submucosa of asthmatic patients [15]. IL-25 was first found in CD4+ T helper 2 (Th2) cells [3], and was later verified not only in immune cells but also in non-immune cells, indicating its various cellular sources. In addition to activated Th2 cells in the gastrointestinal tract and other mucosal tissues, immune cells including bone marrow derived mast cells, alveolar macrophages and eosinophils are also sources of IL-25 [16, 17]. For non-immune cells, epithelial cells in the respiratory and digestive systems, brain capillary endothelial cells and fibroblasts can also secrete IL-25 [18, 19]. A recent study showed that mesenchymal stem cells from the placenta and bone marrow also secrete IL-25 [20].
Studies show that apart from Th2 cells, a rare population of epithelial cells that are recognized as tuft cells is an important source of IL-25 in the intestinal environment [21]. Tuft cells are a group of rare secretory epithelial cells mainly distributed in the respiratory tract and gastrointestinal tract [22]. In the gastrointestinal tract, tuft cells are found throughout the simple columnar epithelia of the stomach, the entire small and large intestine, and the pancreaticobiliary system, accounting for 0.4% to 2% of the epithelial cells in the intestine [23, 24]. Tuft cells act as proficient chemosensory sentinels and produce biological effector molecules, including IL-25 [22, 25]. Tuft cells respond to a wide variety of substances in the gastrointestinal tract by expressing various receptors. Although not fully understood, tuft cells are known to express Tas1Rs, Tas2Rs, and SUCNR1 for sweet substances, bitter substances, and succinate, respectively [26, 27]. Moreover, tuft cells are a group of epithelial cells that depend on the development and expression of the transcription factor POU2F3 [23]. Decreased levels of tuft cells and IL-25 appear in the intestine of POU2F3-/- mice compared to wild-type (WT) mice during parasite infection [28]. Notably, Zhao et al. found that in the small intestine, epithelial cells are the main cell sources of IL-25, rather than immune cells [29]. Recently, von Moltke et al. further identified that in the model of mice infected with helminths, tuft cells of 4 epithelium cell types in the small intestine constitutively expressed IL-25, and all IL-25+ cells were tuft cells, suggesting that tuft cells are the exclusive epithelial cell source of IL-25 in the small intestine [30]. The above evidence suggest that tuft cells are the only cellular sources of IL-25 in the small intestine. However, the knowledge of tuft cells is still limited and further investigation into their involvement in different diseases is required.
Receptors of interleukin 25
The IL-25 receptor (IL-25R) is composed of two subunits, IL17RA and IL-17RB. IL-25R is expressed in various respondent cells, including memory Th2 cells, Th9 cells, airway smooth muscle cells, endothelial cells, eosino- phils, basophils, and group 2 innate lymphoid cells (ILC2s) throughout skin, brain, airway, and intestine tissues [17]. In the intestinal environment, IL-25R is mainly expressed on the surface of ILC2 and CD4+ Th2 cells, which produce and secrete type 2 cytokines such as IL-4, IL-5, IL-9, and IL-13 in response to IL-25 [31].
Interleukin 25 impacts the differentiation of initial CD4+ Th2 cells into Th2 cells [8]. Moreover, IL-25 binds to the IL-25R expressed by Th2 cells and induces secretion of Th2 cytokines [3]. Paradoxically, the effect of IL-25 on Th2 cells in the generation of adaptive type 2 immune responses seems controversial. A study showed that IL-25R is required for late effector responses against chronic infection instead of the generation of a sufficient Th2 response to helminth infection [32]. Mearns et al. also suggested that IL-25 is unable to impact the differentiation of Th2 cells and their development into effector or memory Th2 cells [33]. However, several studies have offered evidence that IL-25 may impact Th2 memory cells [34, 35].
In addition to Th2 cells, ILC2s, a group of natural adaptive lymphocytes, are also important downstream cells of IL-25 [36]. ILC2s were initially described as IL-4-, IL-5-, IL-13-producing non-B-/non-T-cells and were later characterized as Lin–c-Kit+Sca-1+ cells that could produce large amounts of type 2 cytokines [3, 37, 38]. ILC2s play crucial roles during the initial response in parasite invasion, allergic reactions, tissue repair, and intestinal homeostasis [9]. Recent studies have classified ILC2s into natural ILC2 (nILC2s) and inflammatory ILC2 (iILC2s), of which only iILC2s answer to IL-25 and produce type 2 cytokines [8, 39].
There are also other IL-25 responsive immune cell types. Blockade of IL-25 signaling results in low levels of natural killer (NK) T-cells and IL-13 production by neutralizing IL-25 and IL-17BR in an oxazolone-induced mouse model of colitis, suggesting that IL-17BR+ NK T-cells are another group of IL-25-respondent cells in the gut [40]. Hongjia et al. found that dendritic cells also carry IL-17RB [41], and Chua et al. subsequently suggested that IL-25 activates dendritic cells in Ruminococcus gnavus-associated intestinal dysbiosis [42]. In addition, eosinophils are also identified as effector cells of IL-25 signaling that are protective against Clostridioides difficile infection [43].
Recent studies have identified several additional intestinal non-immune cell types, such as epithelial cells themselves and mesenchymal cells [44, 45]. In a study focused on inflammatory bowel diseases, LGR5+IL-25R+ cells increased in the inflamed mucosa in active patients, indicating that intestinal mesenchymal stem cells (MSCs) are possible receptors to IL-25 [45]. IL-17RA and IL-17RB are also expressed compositionally in epithelial cells and smooth muscle cells [29]. Exogenous IL-25 administration up-regulates the expression of both IL-25 itself and its receptor IL-17RB, which is similar to the phenomenon in nematode infection, suggesting a positive feedback mechanism that significantly amplifies the effect of IL-25 during infection [29]. IL-25 induces contraction of intestinal smooth muscle cells, which facilitates the excretion of worms during nematode infection [29]. The cellular mechanism of IL-25 promoting tracheal smooth muscle contraction in mice also has been identified in recent studies [46]. The function of IL-25R and its interaction with other cytokines in the intestine require further elucidation as receptors of IL-25 could be potential targets in treating intestinal diseases.
Signal transduction
Given the fact that IL-25 plays an essential role in the pathology of multiple diseases, an understanding of signaling pathways of IL-25 is indispensable (Fig. 1). Downstream signaling cascades of IL-25 include nuclear factor kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and janus kinase/signal transducer and activator of transcription (JAK/STAT) [2].
Fig. 1
Signaling pathways of interleukin 25 (IL-25). IL-25 forms a homodimer 2:2 complex with IL-17RB with two “wing-like” IL-17-RA co-receptors on both sides. Act1 is recruited by the SEFIR domain of IL-17R (both IL-17RA and IL-17RB). TRAF6 binds to Act1 and mediates NF-κB and MAPK signaling. NF-κB signaling is mainly TRAF6-dependent while activation of MAPKs is also TRAF4-dependent. TRAF4 recruits Smurf2 and leads to disassociation of DAZAP2 from IL-17RB, increasing Act1/IL-25R interaction and IL-25 reactivity. In addition to NF-κB and MAPK signaling, IL-25 also activates the JAK/STAT pathway
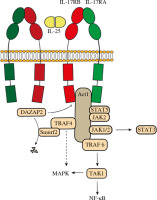
As a unique member of the IL-17 cytokine family, IL-25 mediates immune activities by binding to the IL-17RA/IL-17RB heteromeric receptor complex [47]. It is reported that IL-17RA binds to the IL-17RB-IL-25 complex instead of directly binding to IL-25 [48]. Recently, Wu et al. confirmed that IL-25 binds to IL-17RB and identified 2 discrete linear and cyclic epitopes to be the primary docks [49]. Wilson et al. further demonstrated that IL-25 only interacts with IL-17RB to allosterically facilitate the formation of the ‘tip to tip’ interface of IL-17RB-IL-17RA, which is a key receptor-receptor interaction required to initiate signal transduction [50]. Moreover, the study identified the IL-25–IL-17RB–IL-17RA ternary complex in which IL-25 forms a homodimer 2:2 complex with IL-17RB with two “wing-like” IL-17-RA co-receptors on both sides [50]. Due to this unique structure, both IL-17RB and IL-17RA are essential for IL-25 signal transduction. Competition between IL-25 and other IL-17 family members against collective receptors (IL-17RA and IL-17RB) may result in the disability of IL-25 [50, 51]. Studies have shown that IL-25 inhibits IL-17 function as an IL-17A receptor antagonist in synoviocytes [52] and IL-25 function can also be inhibited by IL-17B in colon epithelial cells [51].
The SEF/IL-17R (SEFIR) domain of IL-17R recruits activator 1 (Act1), which also includes a SEFIR domain [53]. The interaction between Act1 and IL-17RB is abolished when the SEFIR domain is deleted in either Act1 or IL-17RB [54]. Zhang et al. revealed structures containing the conserved α-helix in both IL-17RA and IL-17RB for Act1 binding [55, 56]. A study determined that Act1 deficiency in intestinal epithelial cells resulted in diminished expression of the Lin–c-Kit+ innate immune cell population as well as Th2 cell-type cytokines and promotion of worm infection. The study further verified that lower expression of IL-25 in intestinal epithelial cells was present in the worm-infected epithelial-specific Act1-deficient mice compared to wild-type control mice, indicating that Act1-mediated signaling in the epithelium is vital for the IL-25-induced Lin–c-Kit+ innate cell population [57].
TNF receptor-associated factor 6 (TRAF6), a member of the TRAF family, binds to the amino-terminal part of Act1[54]. The specific structure of the Act1–TRAF6 complex remains vague, yet 3 TRAF6-binding motifs in Act1 (amino acid residues [in human Act1] 15–20 and 37-42 in the N-terminal region and 327-334 in the Ser–Gly–Asn–His hydrolase region, respectively) have been discovered [54]. Recruited TRAF6 subsequently activates TAK1, then leading to NF-κB and MAPK-AP-1 (JNK and p38) pathways [58]. TRAF6 has been proven to be vital for the NF-κB pathway but dispensable for the MAPK pathway, as NF-κB activation was diminished in TRAF6-deficient murine embryonic fibroblasts, while MAPK activation was not [46, 59]. This also suggests the existence of MAPK activation pathways independent of TRAF6. TRAF4 is also a member of the TRAF family recruited by Act1 [60]. TRAF4 knockout mice showed abolished IL-25-induced phosphorylation of ERK1/2 and p38, revealing that activation of MAPKs is TRAF4-dependent [61]. On the other hand, TRAF4 mediates the recruitment of smad-ubiquitin regulatory factor 2 (Smurf2), which induces degradation of azoospermia-associated protein 2 (DAZAP2), an inhibitory molecule that blocks Act1/IL-25R interaction by interacting with IL-17RB [61, 62]. The disassociation of DAZAP2 from IL-17RB increases Act1/IL-25R interaction and IL-25 reactivity [61].
Interleukin 25 also activates JAK/STAT, which is responsible for inducing a variety of cellular mechanisms. STAT5 is recruited to IL-25R in a ligand-dependent manner via unique tyrosine residues on IL-17RB, suggesting that IL-25 activates STAT5 in a direct way independent of Act1 [63]. JAK2 and STAT5 bind to the IL-25R complex upon IL-25 stimulation [64]. IL-25 also stimulates keratinocyte proliferation and induces the production of inflammatory cytokines and chemokines by mediating the Act1-JAK1/2-STAT3 pathway through IL-17RB in keratinocytes [5].
Diverse signaling pathways activated by IL-25 provide possible therapeutic targets for related diseases and are a direction for future research. However, the specific mechanism mediating IL-25 signaling requires elaboration.
Interleukin 25 in intestinal diseases
Intestinal helminth infection
Parasitic helminth infection elicits a type 2 cytokine-mediated inflammatory response, in which IL-25 plays the role of mediating type 2 immunity. IL-25 is considered essential for clearing helminth infections. Wild-type mice were resistant to Trichuris infection with the expulsion of worms by day 20, whereas IL-25–/– mice failed to eradicate the infection [65]. Similar results indicating impaired expulsion of Trichinella spiralis [66], Nippostrongylus brasiliensis [33, 67], and Heligmosomoides polygyrus [34] worms were found in mice lacking IL-25. IL-25 also inhibits intestinal inflammation caused by helminth infections. IL-25–/– mice infected by Trichuris showed intestinal inflammation associated with increased expression of interferon γ (IFN-γ) and IL-17 [65]. IL-25 reduces Th1 cytokines such as IL-1, IL-6, and TNF-α produced by most helminth infections [21].
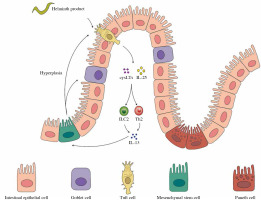
Tuft cells have been lately identified as the major cellular source of IL-25 in the small intestine upon helminth infection [28, 29]. Tuft cells employ a chemosensing pathway to sense lumen cues, such as microbe-derived metabolites [68]. Following the release of IL-25 from tuft cells there occurs rapid and robust expansion and production of cytokines secreted by ILC2s [28, 67, 69]. Th2 cell responses and clearance of N. brasiliensis infection were significantly compromised in ILC2-deficient (Rorαfl/sgIl7rCre), demonstrating the necessity of ILC2s during nematode infection [70]. Recently, a feed-forward circuit (Fig. 2) involving tuft cells, IL-25, and ILC2 was discovered during helminth infection in the small intestine. IL-25 derived from tuft cells activates ILC2s to secrete IL-13 and IL-4, which induce epithelial crypt progenitors to promote differentiation of tuft and goblet cells in reverse [30]. The circuit was further addressed in driving small intestine remodeling, the activation of which by chronic administration of IL-25 as well as H. polygyrus infection led to alterations in the small intestine, including the increase of secretory cells, a corresponding decrease of absorptive enterocytes, and lengthening of the small intestine, resulting in metabolic homeostasis in the host and a reproductive niche for the pathosymbiont [68]. The study also illustrated succinate, an end-product of Tritrichomonas metabolism detected by tuft cells, as a signal of hypoxic stress and injury to initiate a tuft–IL-25–ILC2 circuit and ameliorate intestinal inflammation. This indicates a potential role of diet and microbiota-derived intestinal metabolites in inducing a tuft–IL-25 circuit during helminth infection, the regulation of which remains to be fully elucidated [68]. Delay in helminth clearance in tuft cell-deficient mice is more significant than that in IL-25-deficient mice, suggesting that the tuft–IL-25 interaction involves more regulation [71]. Indeed, analysis of the tuft cell–ILC2 circuit further demonstrated that tuft cells synthesize and secrete cysteinyl leukotrienes that cooperate with IL-25 to activate ILC2s specifically during N. brasiliensis infection; however, cysteinyl leukotrienes seem to be dispensable for the tuft cell response induced by intestinal protists [71].
Fig. 2
The feed-forward circuit in gastrointestinal helminth infection. Tuft cells are the major cellular sources of interleukin 25 (IL-25) in the small intestine upon helminth infection. A feed-forward circuit involving tuft cells, IL-25 and ILC2 appears during helminth infection in the small intestine. IL-25 derived from tuft cells activates ILC2s to secrete IL-13 and IL-4, which induce epithelial crypt progenitors to promote differentiation of tuft and goblet cells in reverse
In primary Echinostoma caproni infection, IL-25 is required for the development of the Th2 phenotype, and IL-25 may also have an impact on Th2 memory cells to resist helminth infection in secondary infection [35]. The exogenous administration of IL-25 restored the severely impaired host memory response against a secondary infection with H. polygyrus bakeri in IL-25–/– mice [34]. Moreover, secondary infection with E. caproni elicits a type-2 response without IL-25 expression [35]. These studies imply the potential function of IL-25 of inducing differentiation of the Th2 memory subset during nematode infection, the mechanism of which still requires investigation.
Though IL-25 is generally considered a protective cytokine during helminth infection, IL-25 may also be involved in the pathogenic process [21]. Disruption of IL-25 along with thymic stromal lymphopoietin (TSLP) and IL-33 signaling suppressed chronic and progressive pulmonary type 2 cytokine-driven inflammation and fibrosis; however, blockade of IL-25 alone failed to present any significant change [73]. A recent study determined that IL-25 level peaked in accordance with oviposition around week 10 after infection. These results suggest that IL-25 might play a role in the maintenance of schistosome egg-induced pathology rather than in initiating schistosomiasis [73].
Inflammatory bowel diseases
Interleukin 25 is also involved in inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD). The etiology and pathogenesis of IBD have not been fully understood. It is suggested that its pathogenesis is associated with the inflammatory response of the intestinal mucosal immune barrier to intestinal antigen [15].
Interleukin 25 production was found to be downregulated in human IBD mucosal samples by analyzing IL-25 RNA and protein expression [74]. Su et al. confirmed that IL-25 levels significantly decreased in the intestinal mucosa as well as the serum of patients with active inflammatory bowel disease and were negatively correlated with endoscopic disease activity and C-reactive protein level. Subsequent in vitro studies demonstrated that IL-25 inhibited IBD CD4+ T-cell activation to negatively regulate IFN-γ, TNF-α, and IL-17A production but enhanced IL-10 secretion [75]. These above findings suggest that IL-25 is down-regulated in IBD.
Although most of the studies related to IL-25 suggest that IL-25 plays a role in inhibiting inflammation, there are studies showing that IL-25 may promote type 2 immune colitis [8]. IL-25 can be enhanced after anti--TNF-α or TGF-β1 treatment, underlining its different regulation by TNF-α and TGF-β1 [74]. Considering that IL-25 can be regulated by different immune response components and that UC and CD are mediated by different immune types [76], we will discuss them separately.
Accumulating evidence has demonstrated that UC is mainly a type 2 immune response in which IL-17RB+ NK T-cells and nuocytes (a subset of ILC2 cells) induce type 2 cytokines, including IL-4, IL-5, and IL-13 [77]. In a tight control follow-up study enrolling serum samples of IBD patients, IL-25 expression was significantly higher in samples from patients with unstable remission compared to those with stable remission and was linked to T-cell activation when restricting analyses to UC patients only [78]. Blockade of IL-25 signaling remarkably improved weight loss and colon ulceration and resulted in decreased nuocyte and NK T-cell infiltration of the mucosa in mice with oxazolone-induced UC [40]. Similar results indicating that IL-25 is pathogenic in UC were obtained in several other studies using dextran sulfate sodium (DSS)-induced colitis models [51, 79]. IL-25 also has a negative effect on monocyte and macrophage production, resulting in amelioration in oxazolone-induced colitis [80]. Interestingly, several other studies offered contradictory results using the same colitis models. The elevation of IL-23 and TGF-β1 along with the promotion of inflammation was seen in DSS + rIL-25 (recombinant IL-25)-treated mice compared to DSS-treated mice [81].
Crohn’s disease is another complex pathogenesis related to Th1 cytokines, including IFN-γ, TNF-α, and IL-23 [82]. It is also considered related to Th17 cells, which produce increased levels of IL-17 and IL-22 [83]. Tuft cells were significantly reduced in CD ileal specimens compared to normal specimens from patients without a CD diagnosis [84]. In a TNF-α-overexpressed mouse model of colitis, tuft cell hyperplasia induced by administration of succinate resulted in reduced ileum inflammation [84]. IL-25 may play a similar protective role as tuft cells in CD as tuft cells are predominant sources of IL-25 in the small intestine. Indeed, restoring the expression of IL-25 in the colon led to inhibiting the Th1/Th17 pathway mediated by IL-12/23 in the mucosa, revealing potential for CD therapy [12]. Inflammation and production of IL-12 and IFN-γ were significantly reduced after IL-25 treatment in mice with peptidoglycan (PGN)-, 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis, indicating that IL-25 inhibits Th1 cell-driven inflammation in the gut [80]. Relying on commensal bacteria, IL-25 is also able to limit Th17 responses through the direct induction of IL-13 in the intestine [85]. Another study verified that IL-25 downregulated CD4+ T-cell differentiation into Th17 and Th1 cells through IL-10, particularly in CD patients [75].
A previous article suggested that IL-25 is a double-edged sword, and in our study, we found that IL-25 facilitates UC while inhibiting CD; however, its action may also be affected by endogenous and exogenous sources [8], a possibility which has not been studied yet.
There are also studies investigating the upstream regulation of IL-25. Though the IL-25 gene is located at 14q11.2 within the CD susceptibility locus [86] and was proved to be associated with CD and UC by analysis of genetic databases [87], no significant difference concerning its coding regions (c424C/A) was found between IBD patients and controls [86]. Thus, other factors involved in the transcription of IL-25 resulting in aberrant amounts of IL-25 in IBD patients have been further investigated. The regulation of microRNA (miRNA) may be involved in the development of IBD as altered microRNA expression has been reported in the serum and mucosa of IBD patients [88]. In a study using the luciferase assay, mmu-miR-135b-5p and mmu-miR-691 were verified to bind to the IL-25 3βUTR, leading to reduced expression of IL-25 in UC [89]. Shi et al. discovered a decrease in IL-25 and a correlated increase in miR-31 in the colons of model mice and CD patients [12]. By target validation analysis in mouse primary cells and human colon cell lines, the study confirmed that miR-31 negatively regulates IL-25 expression by binding to its messenger RNA 3'-untranslated region [12]. Restoration of colonic IL-25 expression through the administration of miR-31 inhibitors showed a therapeutic effect for TNBS-induced colitis, suggesting that anti-miR-31 may be a promising therapeutic option in CD [12].
Compared to IBD patients in remission and healthy controls, the CD4+IL-25R+ as well as LGR5+IL-25R+ cells of active CD and UC patients were significantly increased in the colonic mucosa, indicating that IL-25 may act on MSCs in IBD as well [45]. Subsequent in vitro studies showed that IL-25 may facilitate the proliferative effect of MSCs on the intestinal epithelium through the PI3K/Akt pathway to maintain epithelial cell homeostasis [9]. IL-25-primed MSCs improved DSS-induced colitis better than MSCs alone via inhibiting the Th17 immune response and inducing a T regulatory cell phenotype, as a decrease in IL-17A+CD4+ cells and an increase in FOXP3+CD4+ cells in the peripheral blood and lamina propria CD4+ cells of DSS-treated rats were found in the IL-25 MSC group compared to the MSC group [90]. Moreover, Fu et al. demonstrated a novel strategy to promote delivery of MSCs to the inflamed colon and their immunosuppressive capability by manipulating them to express CX3C chemokine receptor 1 and IL-25 in DSS-challenged mice [91]. These findings may shed light on future possible therapies of IBD via targeting IL-25 R+ MSCs or manipulating MSCs to express IL-25.
In addition, anti-IL-25 antibodies and anti-IL-17BR antibodies have been confirmed to reduce the continuing inflammation of IBD [40]. Overall, IL-25 may be a potential therapeutic target for IBD, but the roles of IL-25 in IBD require further investigation (Table 1).
Table 1
Studies on the relationship between inflammatory bowel disease (IBD) and interleukin 25 (IL-25)
| Disease | Study model | Sample | Expression of IL-25 | Other conclusions | Reference |
|---|---|---|---|---|---|
| UC and CD | Human | Serum samples | Highly expressed in UC patients with unstable remission compared to those with stable remission | – | [78] |
| UC and CD | Human and murine | Histopathological samples | Downregulated in both UC and CD patients | IL-25 resulted in amelioration in PGN-, TNBS-, and oxazolone-induced colitis models | [80] |
| UC and CD | Human | Serum samples and histopathological samples | Downregulated in the sera and inflamed mucosa of patients with active IBD compared with controls | IL-25 downregulated CD4+ T cell differentiation into Th17 and Th1 cells through IL-10 in IBD patients | [75] |
| UC and CD | Human | Histopathological samples | Downregulated in both UC and CD patients | IL-25 can be enhanced after anti-TNF-α or TGF-β1 treatment | [74] |
| UC | Murine | Histopathological samples | Overexpressed in oxazolone-induced colitis model | Blockade of IL-25 signaling remarkably improved weight loss and colon ulceration in oxazolone-induced colitis model | [40] |
| UC | Murine | Histopathological samples | – | IL-25-/- mice suffered less weight loss, diarrhea, and shortening of colon length than WT mice after exposure to DSS | [79] |
| UC | Murine | Histopathological samples | – | DSS + rIL-25-treated mice showed promoted inflammation compared to DSS-treated mice | [81] |
| CD | Human and murine | Histopathological samples | Downregulated in the inflamed sites of CD patients and TNBS-induced colitis model | Restoring colonic IL-25 expression via intracolonic administration of MiR-31 inhibitor was therapeutic | [12] |
Food allergy
Food allergy (FA) is primarily a type 2 cytokine disorder and IL-25 has been reported to play a role in epithelial pathogenesis [92]. Indeed, IL-17RB–/– mice, which displayed reduced acute diarrhea, Th2 responses, and Evans blue dye in the intestine [93], were more resistant than iIL-25Tg mice in developing an experimental FA [94]. FA results in the upregulated production of IL-25, IL-33, and TSLP and the activation of NF-kB signaling [95]. Released upon allergic sensitization, IL-25 enhances ILC2-derived IL-13 production, which facilitates goblet cell hyperplasia and intestinal permeability and mediates gut barrier function [94]. The injection of monoclonal antibodies either to IL-25, IL-33 receptor, or TSLP strongly inhibited FA development, and optimal suppression of established FA required a cocktail treatment with all 3 monoclonal antibodies [96]. This study provides proof that IL-25 is imperative to induce FA and sufficient to maintain this disorder at least partially. Moreover, IL-25 seems to be the bridge that crosstalks between the skin and gut to propagate allergic reactions. Upon mechanical skin injury, intestinal cell-derived IL-25 along with keratinocyte-derived IL-33 increase and activate ILC2 to produce IL-4, thus promoting expansion and activation of mast cells in the intestine, suggesting that inhibition of scratching may be therapeutic in alleviating FA by reducing intestinal mast cells [97]. Oxytocin may also be a novel treatment for FA by suppressing the production of TSLP, IL-25, and IL-33 through NF-κB signaling [98]. OXTR–/– mice, which were abolished by oxytocin receptor (OXTR), showed extreme increases in TSLP, IL-25, and IL-33 levels as well as severe systemic anaphylaxis and intestinal inflammation [98].
Colorectal cancer
In cancer, IL-25 also plays a paradoxical role which includes both tumor supportive and tumor suppressive effects. IL-25 was shown to exert anti-cancer effects in a few cancers including colon cancer, dependent on B cells and increased levels of eosinophilia induced by IL-5 [99]. IL-25 and its receptor showed a significant decrease in tissues of colorectal cancer (CRC) patients compared with ulcerative colitis patients [100]. Acute blockade of IL-25 led to increased risk of tumor progression and tumor burden in a murine colitis-associated cancer model [101]. These results indicate that IL-25 and IL-25R might tend to inhibit CRC in the long-term inflammatory environment such as ulcerative colitis. However, there is evidence that IL-25 may be tumor supportive via promoting the cell cycle, inducing epithelial-mesenchymal transition and metastasis [102]. In CRC patients, systemic levels of IL-25 were significantly elevated in stage IV patients compared to stage I-III patients [103]. A recent study identified that CRC patients with higher tumor IL-25 expression had reduced survival and that the IL-25-ILC2 axis was a possible pro-tumoral mechanism of CRC. In the Apc1322T/+ mouse model of spontaneous intestinal tumorigenesis, mice were treated with rIL-25 for eight weeks, and an increase in tumor burden was observed. This result was due to dramatically increased tumor infiltrating ILC2s affected by IL-25 preferentially. Moreover, IL-25-deficient Apc1322T/+ mice developed fewer tumors and longer life expectancy than IL-25-replete controls, indicating the IL-25-ILC2 axis as a novel therapeutic target against CRC [104]. Though IL-25 shows potential for future cancer treatment, analyses regarding levels of IL-25 and its receptor at each stage of diseases and the mechanisms involved are required.
Conclusions
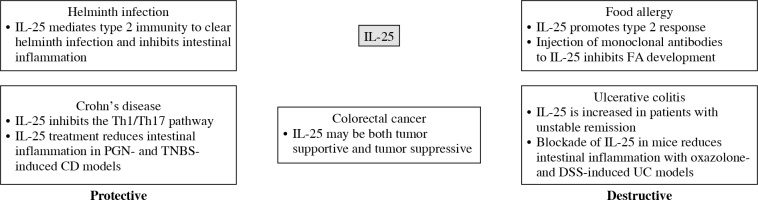
Interleukin 25, a unique member of the IL-17 family, is considered a type 2 cytokine and mediates crosstalk between adaptive and innate immune responses. In this review, we focused mainly on intestinal diseases despite its multiple functions in other systems. Existing evidence suggests that IL-25 plays a dual role in immune responses during the development of different intestinal diseases. IL-25, produced as the first line of defense against infections and stimulations, promotes type 2 cytokines, including IL-4, IL-5, and IL-13, produced by both immune and non-immune cells. This role of IL-25 is vital in the pathogenesis of FA and UC as well as in the adaptive remodeling and clearance during helminth infection. Another role of IL-25 is inhibiting Th1 and Th17 inflammation in the gut; thus, it may be protective in CD. Finally, IL-25 shows potential in the prognosis and treatment of colorectal cancer. A summary of protective and destructive effects of IL-25 in intestinal diseases is shown in Figure 3.
Fig. 3
Protective and destructive functions of interleukin 25 (IL-25) in intestinal diseases are briefly summarized
CD – Crohn’s disease, PGN – peptidoglycan, TNBS – 2,4,6-trinitrobenzene sulphonic acid, FA – food allergy, UC – ulcerative colitis, DSS – dextran sodium sulphate
Though a couple of mechanisms of IL-25 involvement in the intestinal environment have been discussed, literature data are still limited. The specific molecular mechanisms of IL-25 action elicited by different stimulants (e.g., helminths, allergens, and inflammation) and how IL-25 is involved in other intestinal diseases (e.g., CRC) need to be further explored. Most studies concerning IL-25 in the intestinal environment are mostly limited to helminth infections, and its relationship with other intestinal diseases needs more attention. Levels of IL-25 in peripheral blood and tissues in patients might be a valuable biomarker for predicting prognosis. And since studies on CRC and IBD have been mainly focused on animal models, it is suggested that these investigations should be more focused on sensitivity and specificity of IL-25 and IL-25R levels in patients. More prospective studies with larger sample sizes are also needed to draw more definitive conclusions.
The idea of IL-25 treating helminth infections is promising, as studies show that IL-25 can improve helminth infections by promoting differentiation of epithelial cells and helminth clearance. Recent investigations have reported that IL-25 may target IL-25R-expressing cancer cells and result in positive clinical responses. It may also be therapeutic for CD via downregulating Th17 and Th1 inflammation in the intestine. Blockade of IL-25 may also be therapeutic in UC where IL-25 is considered pathogenic. Studies that evaluate the clinical application of humanized anti-IL-17RA antibody may offer indirect evidence of the inhibition of IL-25 in clinical settings [105, 106]. Nevertheless, data on the direct inhibition of IL-25 for the treatment of intestinal diseases are still scarce and require further investigation [107]. The structure, function, target and potential mechanism of IL-25 still require further investigation for drug development. It is hoped that an IL-25-based therapeutic approach may be promising in the treatment of intestinal diseases.


