Purpose
Brachytherapy utilizes the property of inverse square law to deliver high-dose to the tumor with a steep dose gradient, hence improving therapeutic ratio. It has been demonstrated as a tangible option in the treatment of head and neck cancers, specifically as a sole modality in early tumors or as a boost to tumor bed. Most tumor sites, such as the lip, tongue, floor of the mouth, tonsil, pharynx, nasopharynx, sinuses, and neck, are accessible for the placement of afterloading applicators and catheters [1]. Perioperative brachytherapy has the advantage of combining catheter placement with surgical resection in carefully selected head and neck cases that are bound to receive adjuvant radiation treatment [2]. Target definition is more comprehensive in these cases due to the process being under direct vision of the patient’s anatomy with more accurate placement of markers. This reduces uncertainty of the target recognition process post-operatively, where changes in patient’s anatomy and tissue edema can complicate the process [2]. Brachytherapy has other advantages, including delivering a higher radiation dose to the tumor while sparing surrounding normal tissue from radiation, reducing the overall treatment time, and obtaining a conformal dose distribution to the desired target [1].
Brachytherapy can be used as monotherapy or as a boost along with external beam radiotherapy (EBRT). It can be applied in primary or recurrent carcinoma of the head and neck, where hollow catheters can be safely inserted. Indications for brachytherapy in post-operative setting include lympho-vascular space invasion, peri-neural invasion, positive or close margins, and lymph nodal involvement in locally advanced head and neck cancers [3]. When combined with EBRT of 40-50 Gy, the brachytherapy boost is generally to a dose equivalent of 30 Gy for adequate local control. Concurrent platin-based chemotherapy is indicated for margin-positive disease and extra-capsular spread [4].
Perioperative or intra-operative brachytherapy, often used interchangeably, is a promising modality that utilizes direct visualization of the structures at the surgery to place brachytherapy afterload catheters. Theoretical advantage of this procedure is that the challenges of post-operative target delineation uncertainty resulting from tissue edema and anatomical defects can be overcome, and a second invasive procedure for catheter placement is avoided. Typically, brachytherapy is delivered with low-dose-rate (LDR), pulsed-dose-rate (PDR), or high-dose-rate (HDR) technique, with HDR dominating treatments in the modern era. Treatment is delivered over 2-5 days in an in-patient setting with catheters in situ. Perioperative brachytherapy series report 5-year loco-regional control rates in the range of 72-93%, depending on tumor volume and extent of the disease; early disease being treated with brachytherapy alone vs. more advanced cases requiring a combination of EBRT with brachytherapy [5, 6].
Common approaches to head and neck brachytherapy
The most common type of head and neck brachytherapy is interstitial brachytherapy, followed by surface applicator techniques and intra-cavitary applications. In interstitial brachytherapy, the radioisotope is placed either temporarily or permanently into the tumor site or bed. Temporary interstitial implants involve the placement of hollow needles or tubes directly into the tissue, into which radioactive sources, such as iridium-192 (192Ir), can be loaded [7]. Several techniques have been proposed for this intricate procedure, including guide gutter or Pierquin technique, Henschke plastic tube method, and hypodermic needle technique of Pierquin. The commonly used plastic tube technique of Henschke is straight or through-and-through, looped around the area of interest, or blind-ended on one side. In the sealed-end technique, the catheters exit through only a single-side of implanted target volume. This technique is generally used for the placement of interstitial catheters after head and neck dissection for residual disease or for recurrent disease. Typically, catheters are approximately placed 1 cm apart in a single-plane implant, 1.2 cm to 1.5 cm in a multiplane implant, in accordance with the rules of Paris system [8].
For tumors of the head and neck invading the base of skull, brachytherapy should be considered if there is considerable doubt about an R0 resection. Proximity to critical structures and complex anatomy make complete surgical resection a challenge [8]. However, implanting catheters in this region can also be difficult while using the standard cranio-caudal approach. Therefore, in the present paper, the technical details of an innovative Palled-Chavan technique of placing catheters through infra-zygomatic process for interstitial brachytherapy to cover the base of skull region were explained, along with its benefits.
Material and methods
Case details
A 42-year-old male patient with a 20-pack/year history of smoking presented with complaints of swelling of his left cheek for 2 months, associated with moderate and diffuse pain over the local site. He was evaluated with a contrast-enhanced chemotherapy (CECT) that revealed an ill-defined heterogeneously enhancing lesion measuring 3.5 cm × 3.1 cm × 3.8 cm involving the upper gingivobuccal sulcus and the left upper alveolar process of maxilla. The lesion was extending to the left maxillary sinus with an erosion of posterolateral and medial wall of maxilla, left lateral pterygoid plate, and left half of the hard palate. Medially, it extended to the left inferior meatus and posteriorly to the retro-antral region and the left retromolar trigone. Additionally, left lateral pterygoid muscle and left infratemporalis muscle were covered by the tumor. Few significant lymph nodes were present in the ipsilateral level 2 of the neck, the largest being 16 mm × 11 mm. Biopsy was suggestive of squamous cell carcinoma, grade 2. The patient was staged as cT4bN1M0 according to the American Joint Committee on Cancer (AJCC-TNM) 8th edition classification. He was planned for 3 cycles of neoadjuvant chemotherapy (NACT) with TPF regimen (docetaxel, cisplatin, and 5-flurouracil) followed by re-assessment for surgery.
The patient showed partial response on CECT with a reduction in tumor size to 3.2 cm × 2.2 cm × 3.6 cm. However, the extensions to bony structures persisted.
Treatment details
The patient was planned for a radical surgery of total maxillectomy, left hemi-mandibulectomy, and left supra-omohyoid neck dissection with reconstruction, followed by adjuvant radiotherapy.
Surgical resection
After securing airway and performing elective tracheostomy under general anesthesia, an incision was given on the left side of the neck extending up to the ipsilateral angle of the mouth, which extended to the left side of the angle of mouth. Sub-platysmal flaps were elevated. Left side levels Ib, II, and III were dissected, preserving facial vessels after ligating to the sub-mandibular gland and submental area. The internal jugular vein was elevated and preserved, and the left parotid duct was ligated. The flap was then elevated up to the inferior orbital rim superiorly, the zygomatic arch laterally, and the nasal cavity medially. Left hemi-mandibulectomy cut was applied with Gigli saw. Infra-temporal fossa clearance was done and the internal maxillary artery was ligated. Superiorly, the tumor was found close to the orbital fat and was resected. Residual tumor tissue was found near the foramen lacerum and petrous apex, which was excised separately, with incomplete clearance as assessed by the surgeon. Specimens were oriented and labeled, formalin-preserved, and sent for histopathological examination.
Brachytherapy catheter insertion intra-operatively
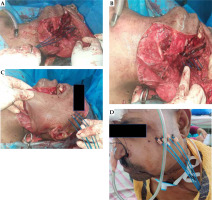
Post-maximal safe resection, placement of catheters was planned based on the extent of residual tumor at the base of skull. Five catheters were initially inserted cranio-caudal with a 1 cm spacing, and a single-plane implant was performed through the lower end of the resected mandible beneath the flap (Figure 1A). The catheters were secured using sutures to the surrounding muscle fibre. We observed that this approach was difficult with improper alignment and incomplete coverage of the post-operative tumor bed. We decided to improvise the technique by re-inserting the catheters through an infra-zygomatic route. The novel Palled-Chavan technique allowed for more horizontal access, and enabled a single-plane implant with 1 cm spacing as originally planned (Figure 1B). Blind-ended catheter was implanted internally with open end outside the patient for connection access to brachytherapy source. The other end was secured into the nasal cavity using buttons. The closed end with buttons was fitted with ties or strings delivered through the nasal cavity for easy removal of catheters post-brachytherapy treatment (Figure 1C, D). The external part of the catheters was chronologically flagged and the patient was moved to intensive care unit for post-operative monitoring. Appropriate antibiotics were administered to prevent infections.
Fig. 1
Operative procedure of the novel brachytherapy technique. A) The initial placement of catheters in the cranio-caudal direction. B) The infrazygomatic approach modified to increase the coverage in the high-risk area. C) Brachytherapy catheters placed 1 cm apart in a single plane. D) Catheters flagged chronologically for easy identification, planning and treatment
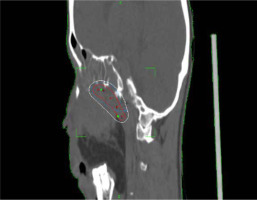
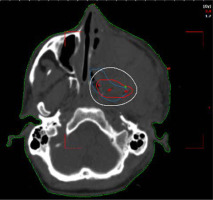
Planning and dose prescription
Planning CT was performed on Philips Medical System Cleveland with 3 mm slice thickness encompassing tumor bed, implanted catheters, and a 5 cm cranio-caudal margin. Target volume was considered gross residual tumor, based on the clinician’s assessment, aided in location by bony landmarks, such as pterygomaxillary fissure, temporal process of the zygomatic bone, greater wing of the sphenoid, occipital condyle, and the lateral nasal wall on the opposite side. High-risk clinical target volume (HR-CTV) was generated from the residual volume with a 3 mm margin, and edited based on patterns of spread. The American Brachytherapy Society recommends a CTV based on clinical parameters. In EBRT, a margin of 5 mm is recommended. Due to the proximity of critical neural tissue and in anticipation of high doses close to the implanted catheters, the margin was limited to 3 mm in this case.
A dose of 27.2 Gy in 8 fractions, corresponding to 3.4 Gy per fraction was prescribed to HR-CTV. Sagi-Nova brachytherapy planning system was applied to generate the plan, and dose distribution was optimized by forward planning altering geographical dwell positions if required to ensure adequate coverage of HR-CTV (Figures 2 and 3), while maintaining organs at risk (OARs) constraints to the eye, optic nerve, and chiasma. CT simulation was repeated daily for catheter displacement or positional changes. Images were satisfactory throughout the treatment and no changes were made. The sagittal and axial views of the plan are shown in Figures 2 and 3.
Treatment delivery and follow-up
The patient was treated with remote afterloading, high-dose-rate brachytherapy using a Cobalt-60 Sagi-Nova device. Two fractions were delivered per day, with a minimum of 6 hours interval between fractions, and the treatment was completed over 4 days. The catheters were removed by tugging at the threaded attachments on the buttoned end, under aseptic precautions.
Post-operative pathology revealed grade 2 squamous cell carcinoma of the left maxillary sinus and upper gingiva. The skull base margin and medial palatal margin were involved by the tumor, and the medial mucosal margin and pterygoid margins were free from the disease, as per an R1 resection status on pathology. Additionally, underlying bone was involved by the tumor and lympho-vascular space invasion was observed. One out of the 23 dissected nodes on the ipsilateral neck was positive for metastasis, with no extra-capsular spread.
The patient was subsequently planned for EBRT and concurrent adjuvant chemotherapy of weekly cisplatin 40 mg/m2 for 5 such cycles. EBRT of dose 36 Gy in 18 fractions was planned for primary and post-operative site of the neck, and 24 Gy in 12 fractions was continued for treatment of the neck. The patient was provided adequate symptomatic and nutritional support during and after the treatment. He is presently on follow-up, and has been disease-free for a period of six months post-treatment.
Discussion
Brachytherapy, as a mode of cancer treatment, is as old as the history of radiotherapy itself, and head and neck tumors were among the first sites to be treated with brachytherapy [7].
Head and neck carcinomas have a higher rate of loco-regional failure, which makes dose escalation important. Brachytherapy alone or in combination with EBRT and chemotherapy leads to local dose escalation, despite improvements in EBRT techniques [4].
Initially, a variety of head and neck sites were treated using rigid radium sources of finite length to deliver LDR brachytherapy. In 1958, afterloading techniques using flexible 192Ir wires have been introduced, reducing radiation exposure to staff, and renewing interest in brachytherapy. This led to the development of new rules of implantation, specifically the Paris system, which then formed foundations of modern day head and neck brachytherapy [9]. Imaging with computed tomography or magnetic resonance has further revolutionized modern brachytherapy into the better and more accurate definition of the target and OARs. The implementation of stepping source technology with the potential for intensity modulation as well as developments in medical and physics quality assurance, have refined the planning process further [4].
The complex anatomy and various subsites of the head and neck region require individual approaches as per the specific sites, using different fractionation and various total dose, subject to clinical requirement [10]. The most frequent sites are the floor of mouth, oral tongue, and oral mucosa, and less common are the oropharynx, nasopharynx, and the base of skull.
Data for use of brachytherapy in head and neck cancers close to the base of skull comes from carcinomas of the nasopharynx. Brachytherapy has shown equivalent local control as that of EBRT boost in instances, where the roof of nasopharynx was irradiated using intra-cavitary applicators close to the base of skull. However, interstitial implants form the majority of head and neck cancer brachytherapy. They can either be temporary or permanent.
In carcinomas involving the base of skull, permanent implants using low-activity 125I seeds have historically been preferred when there was a residual gross disease or involvement of vital structures, which may not be resectable. In the modern era, there has been an increase in the utility of afterloading devices and temporary implants due to easier application, the ability to optimize plan post-implant, and reduced exposure to radiation staff.
Interstitial applications involve loading sources (usually 192Ir) into catheters implanted into the tumor volume. This allows for deliberate and accurate placement of the catheters, and optimization of the implant dosimetry using three-dimensional (3D) planning systems [7]. The Paris system and imaging studies are applied in determination of the placement, number, and spacing of the catheters.
The GEC-ESTRO Head & Neck Working Group published recommendations stating the target definition of gross tumor volume (GTV) as the primary tumor volume defined by clinical examination, aided by imaging and intra-operative findings. Clinical target volume is the GTV plus a safety margin, taking into account possible microscopic extension, which in most cases ranges between 0.5 cm and 1 cm. CTV and planning target volume (PTV) are considered equal in interstitial implants, owing to minimal or no setup uncertainties next to the implant [4]. The other systems of interstitial brachytherapy in use are the Manchester and New York, which include a set of rules describing how the radiation sources have to be distributed to achieve acceptable dose homogeneity. The dose calculation method and the system of dose prescription in common use are TG43 and TG186. The recommended doses for squamous cell carcinomas by LDR or PDR treatments are 45-60 Gy when brachytherapy is applied as the only modality, and 22-29 Gy when brachytherapy is coupled with 47-60 Gy of EBRT post-operatively [4]. EBRT often accompanies brachytherapy following surgical resection for larger tumors, which locally invade critical structures and are difficult to resect without losing their functions. Hence, intra-operatively, surgeons and radiation oncologist can collectively decide to use brachytherapy in such circumstances.
Tumors that are present in critical areas where the surgeon cannot access the entire tumor, such as in the base of skull, brachytherapy has been used with permanent 125I and 10 mci seed placement, both adjuvantly and in cases of recurrences.
In ISBT technique, catheters are placed through a cranio-caudal approach, and this is routine procedure. In our institute, the approach has been changed, and Palled-Chavan technique, which employs an infra-zygomatic approach was shown to have better coverage of residual volume, providing easier access to the site of interest. This technique is best suited for the base of skull residual tumor bed for irradiation as radical as well as boost, post-operatively for better local control. Depending upon the difficulty in placing these catheters at the site of tumor using different approaches and techniques can be further advanced.
Conclusions
Interstitial brachytherapy is used for head and neck tumors as radical treatment as well as boost post-EBRT. The coverage of tumor bed post-operatively sometime becomes difficult in selected areas, including the base of skull. The infra-zygomatic approach is a suitable alternative to the cranio-caudal approach, and can be considered in difficult cases.