Introduction
Autosomal dominant hyper-IgE syndrome (AD-HIES; OMIM 147060) is an inborn error of immunity (IEI) characterized by increased susceptibility to infections, chronic eczematoid dermatitis, and elevated levels of serum immunoglobulin E (IgE) [1-3]. Affected patients are particularly susceptible to infections with Staphylococcus aureus and Candida albicans and display chronic mucocutaneous candidiasis. A hallmark of AD-HIES is a poor tissue inflammatory response resulting in cold abscesses. Additional clinical abnormalities include coarse facial features, abnormal dentition with retention of the primary teeth, cranial synostosis, scoliosis, hyperextensibility of joints, osteoporosis, pathological fractures, central nervous system abnormalities and various vascular malformations including coronary artery aneurysms [2-4]. AD-HIES is caused by heterozygous loss-of-function (LOF) mutations in the signal transducer and activator of transcription 3 (STAT3) [5-7]. STAT3 is a central regulator of immune homeostasis, and as a key effector of T helper (Th) 17 cytokines and Th17 differentiation it plays an essential role in eliminating extracellular bacteria and fungi [8-10]. The immunological phenotype includes a high serum concentration of IgE, eosinophilia, a low number of Th17 cells, and B memory cell lymphopenia [10]. Serum IgE tends to decline with age, particularly in adult patients [10, 11].
The characteristic facial appearance in AD-HIES typically develops by late adolescence and is characterized by facial asymmetry, a prominent forehead, deep-set eyes, increased inter-alar width (IAW), a broad nasal bridge, a swollen lower lip, and coarse facies with dryness and prominent pores [4]. Oral manifestations include the retention of primary teeth and delayed eruption of permanent teeth [11-15]. Approximately 70% of patients with AD-HIES have delayed exfoliation of three or more primary teeth [11]. Prolonged retention of primary teeth can lead to permanent teeth impaction or formation of double rows, in which succedaneous teeth erupt lingual to the deciduous teeth and predispose to malocclusion. Importantly, permanent teeth erupt normally if retained primary teeth are extracted around the physiological exfoliation age [16]. An abnormal number of primary teeth has also been reported [17]. Further oral manifestations include ectopic eruption or retention of permanent teeth, double dentition, a high-arched palate, severe caries, mucosal plaques, and fissures of the tongue and the palate [12]. Asymptomatic palatal and tongue lesions of the oral mucosa and gingiva may develop in more than 75% of patients [15].
We describe here various oral, dental, and maxillofacial abnormalities in 14 Caucasian patients from Central and Eastern Europe with genetically defined AD-HIES. Recommendations for optimal dental care in AD-HIES are also provided and the crucial role of dentists in early diagnosis is emphasized.
Material and methods
Patients
Inclusion criteria were the characteristic clinical findings of AD-HIES and a confirmed disease-causing STAT3 mutation. A total of 14 patients (8 females and 6 males) from 11 families were examined, with a mean age ±SD of 20.07 ±12.24 years (range 6 to 45 years). Patients 3, 4 and 5, and patients 12 and 13 were from the same family (Table 1). The diagnosis of AD-HIES was made by the analysis of clinical, immunological, and radiological findings, and in all cases, by genomic DNA sequencing. Patients were referred to the following hospitals: Department of Pediatrics, University Hospital “Aleksandrovska”, Sofia, Bulgaria; Department of Clinical Immunology and Allergology, St Anne’s University Hospital, Brno, Czech Republic; Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Hungary; Department of Immunology, Children’s Memorial Health Institute, Warsaw, Poland; Division of Pediatric Allergy and Immunology, Necmettin Erbakan University, Konya, Turkey; Department of Pediatric Immunology and Rheumatology, Western Ukrainian Specialized Children’s Medical Center, Lviv, Ukraine. Informed consent was obtained from the patients or their parents.
Table 1
Demographics, genetic data and IgE level of patients with hyper-IgE syndrome (HIES)
Immunochemistry and mutational analysis
Serum immunoglobulin isotype levels were determined by standard immunological assays. For mutation analysis, EDTA blood was obtained, and genomic DNA was isolated using a QIAamp DNA Blood Mini kit (QIAGEN GmbH, Hilden, Germany). All 23 exons and exon/intron boundaries were amplified by PCR using a single pair of primers available upon request. Amplicons were purified and PCR products were sequenced in double strands using the BigDye Terminator Cycle sequencing kit and an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Extraoral and intraoral examinations
For facial manifestations, IAW was measured and compared with published, ethnic group-specific standard values [18]. The presence of a prominent forehead was also registered. The temporomandibular joint (TMJ) was examined according to standard methods [19]. Radiographic imaging including lateral and frontal cephalometric tracings, conventional panoramic X-ray and periapical imaging was carried out to evaluate delayed tooth eruption and TMJ involvement. A Canon EOS 70D camera was used with a Canon macro lens EF (100 mm), and with a Canon circular flash for taking standardized extraoral and intraoral photographs. Extraoral photographs consisted of frontal and profile views. The intraoral set of shots consisted of frontal, upper and lower occlusal views.
Disorders of the palatal, lingual and buccal regions were defined as described previously [15]. Hard palate lesions were classified into four groups (P1, P2, P3, and P4) as follows: P1, mild hyperkeratosis with shallow fissures or classical cobblestone appearance; P2, midline fibrotic thickening shorter than the whole length of the palate; P3, midline thickening all along the palate midline, sometimes surrounded by small papules; P4, multilobular midline thickening with surface papules and nodes. Tongue lesions were classified according to the modified Farman’s parameters [15] of the fissured tongue as follows: T1, superficial (< 1 mm) dorsal tongue lesions which do not extend beyond one-third of the tongue; T2, involvement of the dorsal surface of the tongue with a depth of fissures (> 1 mm) extending beyond one-third of the tongue; T3, the grooves (> 1 mm) extended beyond one-third of the tongue; T4, very deep clefts with or without overlying flaps or fissures along the midline in front of the circumvallate papillae. In one previous study, the latter was referred to as median rhomboid glossitis [12]. Buccal and lip mucosal lesions were individually classified into two sub-groups as follows: B1/L1, unilateral or bilateral striated or patchy hyperkeratotic lesions with or without surrounding erythema; B2/L2, unilateral or bilateral multiple mucosal fissures [15].
Dental charting included calculation of dmft (sum of decayed + missing + filled primary teeth) if primary teeth were presented in the dentition, and DMFT (sum of decayed + missing + filled permanent teeth) and number of MT (number of missing permanent teeth). Periodontal charting was also done including GM-CEJ (distance between gingival margin and the cementoenamel junction in mm) and CPD max (clinical probing depth maximum in mm = the depth of the deepest pocket), measured using a periodontal probe (UNC type). Gingival BOP (bleeding on probing = clinical sign of inflammation) was assessed in only 12 patients due to a lack of cooperation of 2 patients [20, 21].
Statistics
Frequencies for categorical values and descriptive statistics for scale variables were counted. As the patient population was relatively small, normality testing was not performed for variables. To test possible relationships, the Spearman correlation coefficient and p-value were calculated for all variables. P was significant if p < 0.05.
Results
Demographic, genetic and immunological data
Demographics, genetic data and IgE levels of the 14 patients from 11 families are shown in Table 1. Eight females (mean age ±SD: 22.13 ±13.74 years) and 6 males (mean age ±SD: 17.33 ±10.46 years) were studied. Seven patients were over 18 years and two of them were under 10 years. Mutational analysis of STAT3 revealed p.H332Y (c.994C>T, patients 3, 4, 5), p.R382W (c.1144C>T, patients 1, 6, 9), p.R382Q (c.1145G>A, patient 11), p.F384Y (c.1151T>A, patients 12, 13), p.S611G (c.1831A > G, patient 14), p.T622I (c.1865C>T, patient 7), p.V637M (c.1909G>A, patient 10), p.Cys328_Pro330dup (c.982_990dupTGCATGCCC, patient 2) and p.D371_G380del10aa (c.1110-3C>G, patient 8). Mutations p.F384Y (c.1151T>A) and p.D371_G380del10aa (c.1110-3C>G) have not been previously described. The co-segregation of the novel p.F384Y mutation with the disease in this family suggested that the p.F384Y mutation is disease-causing. Furthermore, the same amino-acid residue was affected by other mutations (p.F384C, p.F384L, p.F384S) in AD-HIES patients, which confirms that this missense change is pathogenic. The cDNA analysis of the novel c.1110-3C>G mutation revealed the deletion of exon11 (30 bp, p.D371_G380del10aa), which suggested that the c.1110-3C>G mutation was disease-causing. All patients had elevated serum IgE levels (Table 1). The highest documented IgE level was 66500 IU/ml in a 12-year-old female.
Extraoral manifestations
All patients were affected by eczema and recurrent respiratory tract infections. Nine out of 14 patients suffered from facial dermatitis and 10 had seborrheic dermatitis with similar gender distribution (Table 2). All patients above the age of 7 had at least partial facial features of AD-HIES (Fig. 1). This feature became more pronounced with age. Facial abnormalities included characteristic coarse facial features with rough facial skin, a prominent forehead, deep-set eyes, a broad nasal bridge, and a wide, fleshy nasal tip. IAW was available in 12 patients. The average adult female IAW was 37.5 mm, whereas in adult male patients it was 42 mm. In both female and male children, the average IAW was 29.3 mm and 26 mm, respectively. A prominent forehead was seen in 10 out of 14 patients. The TMJ disorder proved to be a less frequent symptom and was observed in 3 out of 12 patients. Of the 3 affected patients, patient 9 (20 years old) had DMFT = 9 (MT = 3), patient 11 (22 years old) had DMFT = 13 (MT = 0), and patient 14 (7 years old) had DMFT = 18. There was no correlation between the TMJ disorder and MT.
Fig. 1
Facial appearance of patients. All patients above the age of 7 had shown at least partial facial features of AD-HIES. Facial features include coarse face, rough facial skin, prominent forehead, deep-set eyes, increased inter-alar distances, and wide fleshy nasal tip. Of the 14 patients, 9 contributed to having photo documentation
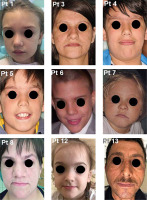
Table 2
Extraoral and intraoral characteristics of patients with HIES
[i] The severity of intraoral disorders (cariological indices, periodontal indices and mucosal involvement) was defined as described in Methods. The severity of the palate and tongue lesions ranged from 1 to 4, while the severity of buccal and lip mucosa ranged from 1 to 2. Bold numbers: IAW values higher than ethnic-specific adult mean values according to Farkas et al., 2005.
[ii] BOP – bleeding on probing, CPD max – clinical probing depth maximum, dmft – sum of decayed + missing + filled primary teeth, DMFT – sum of decayed + missing + filled permanent teeth, F – female, Fam – family, GM-CEJ – gingival marginal and cementoenamel junction, IAW – inter-alar width, M – male, MT – number of missing teeth, N – not present, NA – no available data, Pt – patient, TMJ – temporomandibular joint, Y – present
Intraoral manifestations
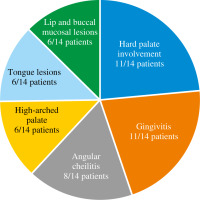
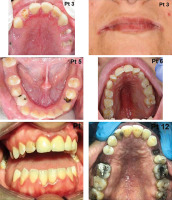
Intraoral abnormalities are summarized in Table 2 and shown in Figs. 2 and 3. Most of the patients (11/14, 78%) exhibited at least one oral lesion. Three patients (2 adults, 1 child) did not exhibit any lesions of the oral mucosa. Two of these patients had skin involvement and angular cheilitis.
Fig. 2
Oral and dental abnormalities. Patient 3: Dental caries affecting roots of all maxillary premolars (14, 15, 24, 25), root of 12 (right upper second incisor) and tooth 22 (left upper second incisor) mesially. Patient 3: Angular cheilitis on both sides with typical erythematous appearance and fissures in their middle. Patient 5: Lower dentition with tongue frenum in the midline. Root of 73 (left primary tooth No 3) persisting distally from intact permanent canine (33). On the right side between the canine (43) and first premolar (44) there is a first primary molar (84) with occlusal carious lesions. First upper permanent molars (36, 46) are missing from both sides. Patient 6: P2 anomaly and high-arched palate. Mixed dentition containing primary teeth 55, 54, 53 and 63, 64, 65. Caries affecting tooth 64 distally. L1 type lip lesion. Patient 7: Total destruction of both upper first permanent molars together with the present primary teeth. Teeth 65, 52, 62 are missing. Patient 8: Mesial caries on tooth 12 (right upper second incisor) and plaque-induced gingivitis (appearing as marginal linear erythema) in both arches. Second premolars (35, 45), first and second molars (36, 37, 46, 47) are missing on both sides; therefore the margin of the tongue becomes visible. Patient 12: No characteristic palatal lesion, but the patient has got a high arched (gothic) palate. The second right permanent incisor (12) has a carious lesion mesially, 15 is missing and the first left premolar (24) appears as a root
In our study, 11/14 of the patients (78%) had palatal lesions. The severity varied from generalized surface keratosis (P1, 5 patients), and a thin midline fibrotic thickening covering less than the entire length of the hard palate (P2, 5 patients) to a thicker linear fibrotic bridge covering the entire length of the hard palate (P3, 1 patient). The most severe P4 lesions (multilobular midline thickening) were not detected. In contrast, in the study by Domingo et al., 55% of the patients had palatal lesions and the proportion of P4 patients exceeded 10% (6/60 pa- tients); however, Domingo et al. examined genetically unconfirmed HIES patients [15]. High-arched palate was found in 6/14 patients (42%). Patients who had more severe palatal lesions (P2 or more severe) had a high arched palate as well. In previous studies the frequency varied from 0% to 71%; however, two of these studies (Grimbacher et al. and O’Connel et al.) included patients with other types of HIES as well as patients without a genetic diagnosis, which can explain the differences between the studies [11, 12, 16, 22]. Tongue lesions were observed in less than half of the patients, 6/14 patients (42%), and the severity ranged from T1 to T2. Tongue fissures affected less than one-third of the dorsal tongue surface, including superficial (T1 in 5 patients) and deep grooves (T2 in 1 patient). The most severe tongue lesions (T2) were found in a 20-year-old male (patient 9) who had multiple deep tongue fissures. No patients had T3 or T4 lesions. In contrast, in Domingo’s study, where 60% of the patients had tongue lesions, T3 and T4 lesions occurred in 10/60 patients (16%) [15]. Lip and buccal mucosal lesions were found in those 6 patients who had tongue lesions as well and included keratotic plaques and/or surface fissures. The most severe forms were B2 and L2, which were found in two patients (14%). Domingo et al. found lower frequency with buccal involvement in 23% and lip involvement in 8% of patients [15]. Angular cheilitis was noted in 8/14 (57%) patients (5 females and 3 males) and none of the patients had a history of or showed clinical signs of oral candidiasis or aphthous ulcers in this patient group. In summary, patients had mild-to-moderate palatal lesions covering P1 to P3, for the tongue only T1 and T2, and for buccal and lip mucosa just level B1/L1 and B2/L2. Patients with higher mucosal scores eventually achieved good oral health due to careful oral hygiene and regular dental follow-ups.
The maximum number of DMFT was 23 (DMFT mean ±SD = 11.18 ±7.58 teeth, min-max: 0-23). Five patients had no missing teeth at all (mean MT ±SD = 5.46 ±5.62 teeth). In 3 patients the number of MT is relatively low (MT in patient 1 = 0, patient 6 = 0, patient 13 = 1) and compared to the age-matched population the DMFT (patient 1 = 1, patient 6 = 1, patient 13 = 1) is also low. We have no information on 2 patients regarding GM-CEJ, CPD max and BOP. GM-CEJ results ranged between 0 and 4 mm (mean ±SD = 1.25 ±0.96 mm) with a minimum of 1 mm and a maximum of 4 mm, whilst CPD max ranged between 1 to 8 mms (mean ±SD = 3.25 ±1.86 mm). Correlations (Spearman rank) between IgE max and CPD max (R = 0.59, p = 0.04) were found. As the junctional epithelium attached at the cementoenamel junction, attachment loss was not detected in any of the patients. Patients had BOP extending from 0% to 90% (mean% of affected marginal surfaces ±SD = 47.83 ±27.86). Clinically if BOP is higher than 10% then the patient has gingivitis. Gingivitis was documented in 10/14 patients (71%).
Primary tooth eruption was unremarkable in all patients evaluated. Primary tooth exfoliation and permanent tooth eruption were on time in all patients. Although there was no delay in permanent tooth eruption in a 12-year-old male patient (patient 4), the lower primary canines were still in place and required extraction. Ectopic eruption of permanent teeth and the retention of permanent teeth over time were not observed. Although tooth agenesis, root resorption, and abnormalities of tooth number or shape have been described in AD-HIES patients, they did not occur in this cohort of patients [23]. Oral ulcers, severe periodontal destruction, oral bleeding diathesis, and benign or malignant intraoral lesions were either not observed during physical examination or were not found in the patients’ previous history.
Discussion
We have studied the intraoral and maxillofacial abnormalities in 14 patients with AD-HIES. The typical face of Job was the most consistent finding though under the age of 7 it was hard to draw reliable conclusions (Fig. 1). Our anthropometric evaluations showed larger- than-average IAW values in all but one adult patient, which corresponds to AD-HIES. IAW measurements also show that in adulthood this distance increases more significantly in male than in female patients. In this study, TMJ involvement was found in patients with low MT and younger age. This underpins the suggestion that TMJ disorders are not strictly related to dental occlusion [24].
In this cohort, intraoral findings confirm that a high-arched palate, gingivitis, angular cheilitis, and mucosal lesions of the palate, tongue, buccal mucosa, and lip can be found in AD-HIES patients (Table 2). It seems that the distribution and severity of any oral lesion were independent of other lesions, age, and gender. The intraoral lesions characteristic of AD-HIES were symptomless, and patients were not aware of them at all. In our study, 78% of the patients exhibited at least one oral lesion. The frequency of oral manifestations was found to be very variable in previous studies. Comparably to our findings, Domingo et al. found that nearly all HIES patients exhibited at least one oral lesion [15]. In contrast, Esposito et al. found nothing abnormal in the mucosal aspect and texture in a 6-year observation of 6 patients with AD-HIES [22]. Similarly, Meixner et al. reported only one patient from a cohort of 13 AD-HIES patients, who had mild abnormal fissuring of the tongue [16].
In our study, angular cheilitis, which is not a specific lesion for AD-HIES, was detected in more than half of the patients. In the general population, it accounts for 0.7-3.8% of oral lesions in adults, and 0.2% to 15% of oral lesions in children [25]. Atopic dermatitis is a risk factor. The reduced bite height resulted in increased saliva at the corners of the mouth, thus softening the epithelium. Infection is secondary to the thinning of the corner epithelium. The causative pathogen behind the cheilitis can be Streptococcus or Candida, depending on age and the number of teeth in place. The more teeth, the greater the solid surface area over which Streptococcus can multiply. However, if teeth are lost, the solid surface area decreases, which allows Candida to become the primary microbe of the mucosal normal flora. If the patient has a normal flora that is shifted towards Candida, then the angular cheilitis is infected by Candida rather than Streptococcus [26].
We found a correlation between IgE max and CPD max which can be attributed to the nature of the disease. Though patients with AD-HIES are not predicted to have a severe periodontal condition (periodontitis), in the case of bad oral hygiene, they may have more pronounced inflammation of the gingiva than patients without AD-HIES, thereby leading to a higher CPD.
Eleven patients showed higher caries prevalence compared to mean values of DMFT in corresponding age groups of WHO dental surveys [27, 28]. Although high caries prevalence is a clinical finding in AD-HIES, it is not limited to it, as it can also be seen in patients with bad oral hygiene and negligence of dental visits. Primary and permanent tooth eruption and primary tooth exfoliation were unremarkable in this cohort, and there were no observed changes in tooth size or pattern. This seems to be an exclusive finding. All previous studies reported a high frequency of persistent primary teeth with delayed primary tooth exfoliation and permanent tooth eruption [11, 12, 15, 16, 22]. It was already shown that STAT3 has a role in amelogenesis, pulp stem cell differentiation, and bone development [29]. The lack of late eruption of permanent teeth or the lack of persistence of primary teeth can be attributed to regular dental care, namely the extraction of teeth to prevent further complications.
STAT3 mutations cluster in the DNA binding and SH2 domains and have been found in many ethnic groups with an equal gender distribution. Despite the different functions of the SH2 and the DNA binding domains and the in vitro observed differences in mutational constructs, overall, the clinical phenotypes were similar and there does not seem to be a significant genotype-phenotype correlation [30, 31]. In some cases, subtle differences in phenotype were observed between patients with DNA binding and SH2 domain mutations. A slight increase of non-immunological features in patients with SH2 domain mutations was reported, including increased frequency of high arched palate, broad IAW, and, in the pediatric sub-group, significant scoliosis [32]. For patients with DNA binding mutations, there was a suggestion of increased mortality [32]. In our study, three patients have SH2 domain mutations. The adult patient (patient 10) had the highest IAW and BOP, but the latter is the result of bad oral hygiene. This patient does not have a high arched palate and had mild mucosal lesions. In the pediatric patients (patients 7 and 14) with SH2 mutation, no scoliosis could be observed.
Conclusions
Although AD-HIES is a rare syndrome, dental practitioners should be aware of the possible oral and dental complications of AD-HIES, and the available treatment modalities. The principal goal of the management of AD-HIES is the aggressive treatment of infections and adequate skin and dental care. Prophylactic antimicrobial therapy for staphylococcal skin infections and sinopulmonary infections is essential. Prophylactic treatment of Candida infections is equally important. While many of the early manifestations of AD-HIES are nonspecific and the penetrance is both age-dependent and incomplete, dentists have a crucial role in early diagnosis. An awareness of the oral and maxillofacial features of AD-HIES may facilitate early diagnosis with genetic counselling and may improve future patient care. Important considerations and recommendations in the management of oral and dental manifestations in patients with AD-HIES must include the following for optimized dental treatment and to prevent dental complications:
– patient education on correct oral hygiene procedures seems crucial to prevent infectious complications;
– regular oral examinations and lifelong complex dental management are important to maintain adequate oral hygiene and to prevent orofacial complications;
– timely extraction of retained primary teeth is important to avoid the impaction of permanent teeth and to prevent the “double dentition” phenomenon, and the development of malocclusion, which is essential to provide effective oral hygiene. To enable spontaneous eruption of permanent teeth in children with AD-HIES, we recommend extracting retained primary incisors when the patient is not older than 9 years of age and retained primary canines and molars when the patient is not older than 13 years of age, after having confirmed the presence of the permanent successor teeth by radiograph [16];
– antibiotic prophylaxis, such as amoxicillin/clavulanic acid, is recommended prior to complex dental treatment;
– provide comprehensive follow-up after procedures to monitor possible complications.