Purpose
Lung cancer is still the most commonly diagnosed cancer and the leading cause of cancer deaths in the world [1]. Due to delayed diagnosis, only 26% of all patients with non-small-cell lung cancer (NSCLC) are alive ≥ 5 years after diagnosis [2]. Advanced NSCLC patients receive limited benefits from historic chemotherapy and radiotherapy, with some severe side effects. Although programmed cell death protein 1/programmed cell death-ligand 1 (PD-1/PD-L1)-targeted therapies prolong survival time, the response rate remains far from ideal dur to underlying resistance [3]. Combination therapy strategies overcome the resistance and bring benefits in terms of overall survival (OS) and progression-free survival (PFS) [4].
Radiotherapy has potent immuno-modulatory effects, and may contribute not only to local control but may also augment systemic anti-tumor immune response. Therefore, the combination of radiotherapy and immunotherapy is a conceptually promising strategy for metastatic NSCLC patients [5]. Limited clinical data indicated that adding stereotactic body radiotherapy (SBRT) to pembrolizumab immunotherapy significantly increased responses and outcomes rates in metastatic NSCLC patients [6]. However, SBRT was associated with non-ignorable radiation-related complications, especially when the lesions were close to the chest wall or mediastinum [7, 8].
Iodine-125 radioactive seed implantation, which contributes to deficiencies of SBRT, can deliver high-dose to the target area while reducing the dose to the normal tissues nearby. It has been adopted in various malignancies, such as prostate cancer [9], pancreatic cancer [10], and lung cancer [11], and it has achieved satisfactory effects without obvious radiation-related complications. A meta-analysis of five randomized clinical trials indicated that combined therapy of iodine-125 brachytherapy and chemotherapy could improve the therapeutic efficacy of advanced lung cancer [12].
However, data about iodine-125 brachytherapy combined with immunotherapy are lacking. Herein, we reported two cases of advanced NSCLC patients effectively treated with computed tomography (CT)-guided percutaneous iodine-125 seed implantation and pembrolizumab after failure of first-line chemotherapy.
Cases presentation and details
This case report was approved by the Institutional Review Board of Hubei Public Health Clinical Center, the central hospital of Wuhan, with ethical number of T20221307.
Table 1 summarizes clinical data of the cases. Computed tomography (CT) images of case 1 after admission on August 15, 2018 showed a mass with cavitation in the right upper lobe, a mass around the right hilum with mediastinum invaded, and stenosis of the right upper bronchus (Figure 1A). Pathology of bronchoscopic transbronchial biopsy confirmed lung adenocarcinoma with negative mutation of driving genes. Brain magnetic resonance imaging (MRI) and positron emission tomography (PET)/CT indicated stage T4N3M1c, IVB with metastasis in supraclavicular/subclavian lymph nodes and mediastinal lymph nodes of 2R, 3A, 4R, and 7, the right pleura, the 7th thoracic vertebrae, and the right adrenal gland. After 4 cycles of first-line chemotherapy consisting of pemetrexed 800 mg (500 mg/m2) combined with cis-platinum 120 mg (75 mg/m2), the effect was evaluated as partial response (PR) with a remarkable reduction of tumor sizes and a relief of the stenosis of the right upper bronchus (Figure 1B). Pemetrexed was then used for maintenance chemotherapy in 16 cycles, and the effect was evaluated as progressive disease (PD) on January 11, 2020, with a new lesion appearing in the right upper lobe (Figure 1C). The patient discontinued treatment for about 3 months for personal reasons, and when he returned to our hospital on April 8, 2020, the tumor in the right upper lobe remarkably increased, together with enlarged mediastinal lymph nodes in 2R and 4R (Figure 1D). No increase in the size or number of metastases was detected. Second biopsy again confirmed lung adenocarcinoma with a PD-L1 tumor proportion score (TPS) of 70% and negative mutations of driving genes.
Fig. 1
Chest computed tomography (CT) images and treatment course of case 1. A) Initial contrast-enhanced CT upon admission. A mass (3.2 cm × 2.6 cm) with cavitation in the right upper lobe, a mass around the right hilum with mediastinum invaded, and stenosis of the right upper bronchus; B) Contrast-enhanced CT after 4 cycles of chemotherapy. The tumors in the right upper lobe and the right hilum nearly disappeared, and the stenosis of the right upper bronchus released; C) Contrast-enhanced CT following 20 cycles of chemotherapy. A new nodule in the right upper lobe (1.4 cm × 1.3 cm); D) Contrast-enhanced CT after discontinued treatment for 3 months. Increased masses (2.1 cm × 2.6 cm, and 3.9 cm × 4.5 cm) in the right upper lobe together with enlarged mediastinal lymph nodes in 2R (2.3 cm × 3.1 cm) and 4R (2.1 cm × 1.7 cm); E) Chest CT following iodine-125 seed implantation combined with pembrolizumab treatment. Almost complete disappearance of tumors in the right upper lobe with only seeds left and remarkable reduction in the size of 2R and 4R lymph nodes; F) Treatment course of the patient
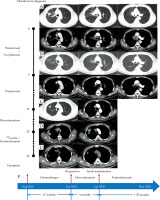
Table 1
Clinical data of the two cases treated with iodine-125 seeds and pembrolizumab
Then, pembrolizumab was applied, and iodine-125 seeds were percutaneously implanted into the tumor in the right upper lobe via CT guidance 2 days after immunotherapy. Post-operative CT showed mild pneumothorax without the need for drainage. The patient completed total course of 35 cycles of pembrolizumab therapy on April, 25, 2022. The effect was maintained as PR at his recent follow-up on May 14, 2022, with almost complete disappearance of the tumor in the right upper lobe, and a remarkable reduction in the size of 2R and 4R lymph nodes (Figure 1E). The patient’s progression-free survival (PFS) was sustained for more than 25 months (Figure 1F). In accordance with the Common Terminology Criteria for Adverse Events version 5.0, there were no treatment-related adverse events, except for rash (grade 1) and hypothyroidism (grade 2), which were treated promptly with no serious consequences.
Case 2 was confirmed with lung squamous cell carcinoma after bronchoscopic biopsy of the mass in the right main bronchus and the left upper bronchus, indicating T4N3M1a, IVA stage and negative mutation of driving genes. The patient’s chest CT after admission on August 14, 2019 revealed a tumor in the left hilum, causing the occlusion of the left upper bronchus as well as tumors in the anterior mediastinum and the left lower lobe (Figure 2A). Bronchoscopy showed 90% stenosis of the right main bronchus due to the tumor obstruction (Figure 2A). Endoscopic procedures were performed using electrocautery snare, argon plasma coagulation (APC), and forceps to ablate the tumor in the right main bronchus on August 27, 2019 and on November 18, 2019. Additionally, 3 cycles of first-line chemotherapy were applied, consisting of docetaxel 130 mg (75 mg/m2) combined with cis-platinum 130 mg (75 mg/m2). On December 17, 2019, the tumors in the left hilum and the anterior mediastinum increased, the tumor in the right main bronchus appeared again as well as a new lesion in the left lower lobe (Figure 2B); therefore, the effect was evaluated as PD.
Fig. 2
Chest computed tomography (CT) and bronchoscopy images and treatment course of case 2. A) Initial contrast-enhanced CT and bronchoscopy upon admission. A tumor (4.8 cm × 6.3 cm) in the left hilum causing occlusion of the left upper bronchus, tumors in the anterior mediastinum (3.1 cm × 4.4 cm) and the left lower lobe (1.2 cm × 1.2 cm) as well as 90% stenosis of the right main bronchus due to tumor obstruction; B) Contrast-enhanced CT and bronchoscopy following 3 cycles of chemotherapy, and bronchoscopic tumor ablation in the right main bronchus. Enlarged tumor sizes in the left hilum (5.3 cm × 7.4 cm) and the anterior mediastinum (7.9 cm × 5.5 cm), and a new lesion in the left lower lobe (1 cm × 1.4 cm). Again appearing tumor in the right main bronchus; C) Chest CT and bronchoscopy, following iodine-125 seed implantation combined with pembrolizumab treatment for 12 months. Almost complete disappearance of the tumors in the anterior mediastinum, the left hilum, and the left lower lobe, with only seeds left in the anterior mediastinum; reduction of the tumor size in the right main bronchus (RMB); D) Chest CT and bronchoscopy at last follow-up; E) Treatment course of the patient
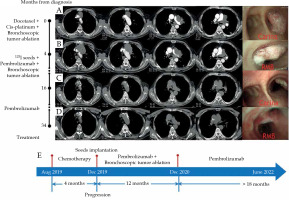
Then, iodine-125 seeds were percutaneously implanted into the tumor in the anterior mediastinum via CT guidance, which caused a complication of mild pneumothorax without the need of drainage. Two days later pembrolizumab was used, and the patient regularly received pembrolizumab therapy every three weeks. The effect was evaluated as PR on December 23, 2020, with almost complete disappearance of the tumor in the anterior mediastinum and the left lower lobe as well as a remarkable reduction in the size of the tumor in the left hilum and in the right main bronchus (Figure 2C). The patient underwent another bronchoscopic tumor ablation on December 27, 2020. Since then, no interventional bronchoscopic therapy was administered, and the patient completed total course of 35 cycles of pembrolizumab therapy. The effect was maintained as PR at his recent follow-up on June 8, 2022, and notably, the tumor in the right main bronchus completely disappeared (Figure 2D). The patient’s PFS has been sustained for more than 30 months (Figure 2E), and survived in healthy status without any treatment-related adverse event, except for mild rash.
CT-guided percutaneous iodine-125 seed implantation
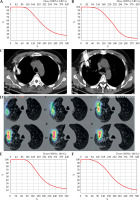
According to the Chinese Expert Consensus [11], the iodine-125 seeds were percutaneously implanted into the tumor via CT guidance, and 0.8 mCi I-125 radioactive seeds (catalogue No.: CIAE-6711; Chinese Atomic Energy Science Institution, Beijing, China) were used. The process was as follows: (1) Pre-operative preparation: CT scans were imported with a slice thickness of 0.35 cm into brachytherapy treatment planning system (BTPS, FTTPS 2.00.00, Beijing Feitianzhaoye Co., Ltd., Beijing, China), and operation field was identified. BTPS simulated the needle path, calculated the number of seeds, and generated a dose-volume histogram (DVH) (Figure 3A and B). The prescribed radiation dose was 140 Gy; (2) Operation: the patient received local anesthesia with 2% lidocaine. 18-gauge needles (Hakko Co., Ltd., Japan) were used, and the intercostal space as a punctured plane was selected. Punctures, according to parameters established by BTPS included puncture site, angle, and depth. After all needle tips reached the distal edge of the tumor, the needle at an equal distance was retraced, and iodine-125 seeds were implanted (Figure 3C); (3) Post-operation: immediate CT scan was taken to verify hemorrhage and pneumothorax. Another CT image within 2 days after implantation was imported into BTPS for post-operative verification (Figure 3D), and an actual DVH was generated (Figure 3E and F). D90 was defined as the dose received by 90% of target volume, and V100 was defined as the percentage of target volume receiving 100% of the prescribed dose. A D90 of at least 90% of the prescribed dose, and a V100 that corresponded to at least 90% of the target volume is the recommended standard of care. D90 and V100 was 142.7 Gy and 91.0% in case 1, and 131.8 Gy and 87.6% in case 2. Both the patients received chest CT or even enhanced CT at 1 month, 3 months, and every three months after implantation, which showed no seed migration.
Fig. 3
Computed tomography (CT)-guided percutaneous iodine-125 seed implantation. Pre-operative dose-volume histogram (DVH) of case 1 (A) and case 2 (B), horizontal axis represents dose, and vertical axis represents volume; C) Seed implantation of case 1. Puncture needles were inserted into the tumor in the right upper lobe under CT guidance, and the distance between puncture needles was 1-1.5 cm; D) Post-operative CT image of case 1 was imported into brachytherapy treatment planning system (BTPS) for verification and showed from inside to outside: 100%, 90%, 75%, 50%, and 25% of the prescribed dose, respectively. Post-operative DVH of case 1 (E) and case 2 (F), horizontal axis represents dose, and vertical axis represents volume
Discussion
Although immunotherapy regimens for advanced NSCLC have improved survival in selected sub-populations, their efficacy remains far from ideal due to underlying resistance; therefore, multimodal combination strategies are needed to optimize their efficacy. Existing data showed that combining radiotherapy with immunotherapy increased responses and outcomes in patients with metastatic NSCLC [6, 13-15]. A retrospective study [13] showed that compared with immunotherapy alone, radiation combination improved 1-year overall survival (OS) (55.7% vs. 59%) in metastatic NSCLC patients. A pooled analysis [6] of two randomized trials (PEMBRO-RT [14] and MDACC [15]) reported that compared with pembrolizumab alone, radiotherapy improved abscopal response rate (19.7% vs. 41.7%), abscopal disease control rate (43.4% vs. 65.3%), median progression-free survival (PFS) (4.4 months vs. 9.0 months), and median OS (8.7 months vs. 19.2 months). The underlining mechanisms might be as follow: radiotherapy can induce multiple immuno-modulatory changes, including upregulation of PD-L1 expression on tumor cells, priming antigen release, and promoting T-cell homing to tumors, which potentially influence the effectiveness of immunotherapy [5]. Results from a mice model demonstrated that radiation enhanced the diversity of T-cell receptor (TCR) repertoire of intra-tumoral T-cells, and increased rates of abscopal responses of metastatic melanoma when combined with anti-CTLA-4 and anti-PD-L1 [16]. Therefore, radio-immunotherapy is a promising strategy for advanced NSCLC patients.
However, the optimal radiation dose fractionated schedule in this combination therapy remains controversial. Pre-clinical data in mice demonstrated that compared with daily fractionated low-dose radiation, high-dose hypofractionated radiation when combined with PD-1 monoclonal antibody, promoted anti-tumor immunity to control primary and distant tumors [17]. MDACC trial also showed that the group that received 50 Gy in 4 daily fractions presented higher abscopal response rate (38% vs. 10%, p = 0.11) and longer PFS times (20.8 months vs. 6.8 months, p = 0.03) compared with the group that received 45 Gy in 15 daily fractions [15]. On the contrary, a study demonstrated that neoadjuvant local low-dose γ irradiation caused normalization of aberrant vasculature and efficient recruitment of tumor-specific T-cells in human pancreatic carcinomas as well as T-cell-mediated tumor rejection and prolonged survival in other immune refractory spontaneous and xenotransplant mouse tumor models [18]. Another mouse study drew similar conclusions, reporting that low-dose irradiation of late-stage tumor-bearing mice, resulted in profound changes in the inflammatory tumor micro-environment, characterized by induction of M1-associated effecter cytokines as well as reduction in pro-tumorigenic and M2-associated effecter cytokines [19]. A retrospective cohort study indicated that higher dose of radiation was correlated with greater risk of grade ≥ 3 lymphopenia, tumor progression, and death after definitive treatment of stage III NSCLC [20]. One systematic review of melanoma patients treated with radiotherapy and ipilimumab suggested that multiple fraction radiation regimens were associated with a more favorable response [21]. The type of tumors, the radiotherapy schedule, and the immune status of patients might explain these discrepancies, and further researches are needed to detect the optimal radiation dose fractionated schedule in this combination therapy. In our cases, continuous low-dose radiation released by iodine-125 seeds indeed promoted immunity to the tumors and produced abscopal effect.
Stereotactic body radiotherapy (SBRT) plays an important role in lung cancer treatment, especially in patients with inoperable, early-stage NSCLC, achieving a high local control rate [3]. However, the external radiation-related adverse reaction cannot be negligible. The risk of grade 3 or above toxicities of SBRT is 4.5% [7], and one of the most common complications is pulmonary dysfunction [22], which could severely impact quality of life of patients with emphysema in our cases. Additionally, for lesions close to the chest wall (< 1 cm from chest wall), as our case 1, and centrally located tumors (lung tumors located within 2 cm around the proximal tracheobronchial tree, or at a maximum distance of 1 cm from the heart and the pericardium, and the esophagus), which were previously acknowledged as a ‘no-fly zone’ for SBRT, as our case 2, SBRT would bring severe complications, such as intense chest pain, bronchial fistulae, hemorrhage, and even death [8].
Implanted iodine-125 seeds can continuously release low-dose γ-rays and X-rays, with a radiation radius of 1.7 cm and a half-life of 59.6 days. Iodine-125 seeds release a cumulative radiation dose of 110-160 Gy in 8-10 months, with only a negligible radiation dose to surrounding tissues [11]; therefore, brachytherapy is a highly precise local therapy, and is superior to SBRT in terms of extremely low-risk of radiation-related adverse reaction [23]. This advantage is more obvious in peripherally and centrally located tumors, just as in our cases. Moreover, different from SBRT, iodine-125 seed brachytherapy achieves the prescription dose by just one procedure, and thus might bring a better treatment experience to patients. Early in 1987, percutaneous iodine-125 seed implantation was performed and achieved satisfactory clinical effects with manageable complications in lung cancer patients [24], and its efficacy and safety were gradually demonstrated. For patients with medically inoperable early-stage NSCLC, CT-guided percutaneous iodine-125 seed implantation had excellent effects on local control of the tumor comparing favorably with SBRT [25, 26]. Iodine-125 seed implantation along with the resected margin for compromised patients with early stage NSCLC undergoing limited resection of lung cancer, resulted in a relatively low incidence of local recurrence and prolonged survival [27]. A meta-analysis enrolled 5 randomized controlled trials, and indicated that for advanced NSCLC, the combined therapy of iodine-125 brachytherapy and chemotherapy could improve the therapeutic efficacy, with significantly better outcomes in complete response (CR), partial response (PR), objective response rate (ORR), disease control rate (DCR), and one-year OS, with risk ratios of 3.66, 1.47, 1.85, 1.19, and 1.46, respectively [12]. Additionally, as second-line therapy, iodine-125 seed implantation combined with chemotherapy compared with chemotherapy only, achieved a better results in terms of overall 2-year local control rate (39.9% vs. 12.5%, p < 0.05), median OS (17.4 months vs. 11.3 months), local median PFS (11 months vs. 7.3 months), and tumor-related chest pain relief (82.1% vs. 30.8%, p < 0.05), and seeds implantation group showed no radiation-related pneumonia, esophagitis, bronchial fistulae, or life-threatening complications [28].
However, seeds implantation, as an invasive procedure, inevitably has puncture-related complications. The risk of pneumothorax was reported to be as high as 47.5%, though only 19.2% patients with pulmonary compression volume > 30% needed invasive closed drainage, and all recovered [26]. Additionally, the treatment depends strongly on personal experience as well as the shape and site of tumors; thus, the quality of implantation is difficult to guarantee. V100 in our case 2 was 87.6%, which was less than the recommended standard of 90%, and in this situation, replantation would be considered. The positions of needles in actual operation in the case 2 was not exactly the same as the pre-plan due to the complex anatomical characteristics of anterior mediastinum and respiratory movement. Difficulty of operation and physical condition of the patient were considered, and replantation of the seeds was not performed. Navigation technology, 3D printing template technology, and robotics technology are expected to further improve the efficiency and accuracy of this treatment [26].
As shown in the present paper, iodine-125 seed implantation combined with pembrolizumab showed satisfactory clinical effects in two patients with advanced NSCLC after a failure of first-line chemotherapy, and the underlying reasons are speculated as follows. Firstly, iodine-125 seeds have a good tumor local control, which was proven to have a powerful association with OS in advanced NSCLC patients [29]. Secondly, the radioactive seeds continuously release low-dose rays, which might effectively amplify the anti-tumor immune response induced by immunotherapy. Last, but not least, iodine-125 seed radiotherapy has negligible radiation damage to surrounding tissues and lymphocytes, and although hemorrhage and pneumothorax are relatively common complications in the process of implantation, they are easily manageable. Consequently, iodine-125 seed implantation contributes to improved quality of life.
Conclusions
In our cases, we found that iodine-125 seeds combined with immunotherapy achieved good local control effect and abscopal result, and the seeds had no significant radiation-related adverse effects due to their characteristics. Therefore, this technique is especially suitable for peripherally and centrally located tumors as well as for patients with emphysema, who are more susceptible to radiation pneumonia. In conclusion, iodine-125 seed implantation combined with immunotherapy is a promising treatment for advanced NSCLC, and more researches are needed to corroborate its efficacy.



