Purpose
Globally, lung cancer (LC) predominates cancer-associated mortalities [1-3]. Approximately 80% of LCs are inoperable due to advanced tumor stage [2]. Systematic chemotherapy and/or radiotherapy are commonly used for inoperable LCs [1-3]. However, many patients cannot withstand such intense and systematic treatment due to older age and/or frail body conditions [4]. Moreover, traditional external radiotherapy is typically correlated with additional adverse effects. Furthermore, radiation dosing can be restricted due to distance of such tumors from neighboring normal tissue and essential organs [5].
Along with the development of interventional therapy, computed tomography (CT)-guided iodine-125 (125I) seeds insertion (ISI) and trans-arterial chemical infusion (TAI) have been widely used for advanced non-small-cell LC (NSCLC) [4-10]. The advantages of interventional therapies include mini-invasive nature and lower treatment-related toxicity. However, clinical efficacy of TAI and ISI alone is limited [4,6,7]. Therefore, many researchers combined TAI and ISI to treat advanced LC cases [11-18]. However, dataset outcomes from an individual investigation could be affected by multiple parameters, thus, a meta-analysis is required to reduce bias and enhance statistical power displayed by reduced cohort size investigations.
Here, we present results of meta-analysis to evaluate the practical effectiveness of combined TAI/ISI in advanced LC.
Material and methods
This meta-analytical investigation complied with preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [19]. Investigational protocol was submitted at INPLASY.COM (INPLASY-2021110058).
Study research
Study selection
Relevant studies were searched in PubMed, Embase, Cochrane Library, CINK, Wanfang, and VIP (until October 2021) databases, using the following keywords: (((((Iodine-125) OR (I125)) OR (125I)) OR (brachytherapy)) AND ((lung cancer) OR (NSCLC))) AND (chemotherapy).
This meta-analysis encompassed the following reports:
Investigation type: comparative studies.
Disease: advanced LC (tumor stage ≥ III).
Types of interventions: TAI with ISI vs. TAI alone.
Languages: not limited.
The following articles were eliminated from this meta-analysis:
Quality assessment
Randomized controlled trials (RCTs) were evaluated using Cochrane risk of bias tool [20]. RCT bias was assessed from performance bias, attrition, detection, selection, reporting, and other sources. Non-RCTs were analyzed with a 9-point Newcastle-Ottawa scale (NOS) [21], with studies exhibiting low, intermediate, or high levels of risk, and receiving scores of ≥ 7, 4-6, and < 4, respectively. Items of NOS included designation (4 points), ability for comparison (2 points), and exposure (3 points).
Data extraction
Two authors retrieved relative data and endpoints separately, and a third researcher resolved any conflict. Baseline data from each publication included first author, publication year, countries, types of design, cancer types, tumor stage, TAI methods, sample size, age, and gender. Outcomes of each study included complete response (CR) rate (CRR), treatment success (TS) rate (TSR), disease control (DC) rate (DCR), one-year/two-year/overall survival (OS) rate, and treatment-related toxicity.
Complete response was defined as complete absence of all target lesions [4,5]. TS was identified as cases of CR and partial response [5]. DC was defined as cases of TS and stable disease [5]. OS was calculated from initial treatment to death. TSR was the primary endpoint in this meta-analysis.
Statistical analyses
RevMan v. 5.3 and Stata v. 12.0 were employed. Dichotomous variables were pooled depending upon odds ratios (ORs) with 95% confidence intervals (CIs), while continuous variables were combined depending on mean difference (MD), with 95% CI. Heterogeneity was assessed by χ2 and I2 tests, with I2 > 50% suggesting significant heterogeneity. Random effects models were employed for significant heterogeneity, while fixed-effects models were employed for significant homogeneity. Heterogeneity sources were analyzed through sensitivity/sub-group assessments. Sub-group analysis was completed based on different cancer types. Funnel plots and Egger tests were utilized for evaluating publication bias risks.
Results
Study inclusion
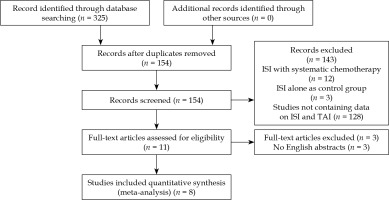
We found 325 relevant studies using the research strategy. After reviewing the abstract and full articles, only 8 studies were included in this meta-analysis (Figure 1). There were 3 RCTs and 5 retrospective studies in the included studies (Table 1). All included studies were from Chinese researchers.
Table 1
Characteristics of the included studies
| Study, year, country [Ref.] | Study design | Cancer type | Group | Sample size | Age (years) | M/F ratio | Stage | NOS |
|---|---|---|---|---|---|---|---|---|
| Guo, 2012, China [11] | RCT | NSCLC | Combined | 103 | 63.4 ±10.1 for all | 151/55 for all | III, IV for all | – |
| TAI alone | 103 | |||||||
| He, 2012, China [12] | Retrospective | NSCLC, SCLC | Combined | 43 | 68 | 28/15 | III: 19 IV: 24 | 8 |
| TAI alone | 65 | 67 | 42/23 | III: 27 IV: 38 | ||||
| Li, 2007, China [13] | RCT | NSCLC | Combined | 15 | 70.5 for all | 19/11 for all | III for all | – |
| TAI alone | 15 | |||||||
| Li, 2014, China [14] | Retrospective | NSCLC | Combined | 24 | 62 | Not given | III: 1 IV: 9 | 7 |
| TAI alone | 32 | 62 | Not given | III: 16 IV: 16 | ||||
| Lin, 2017, China [15] | Retrospective | NSCLC | Combined | 34 | 45-82 for all | 46/24 for all | IIIb, IV for all | 7 |
| TAI alone | 36 | |||||||
| Xing, 2011, China [16] | Retrospective | NSCLC, SCLC | Combined | 57 | 59 for all | 58/44 for all | III, IV for all | 7 |
| TAI alone | 45 | |||||||
| Zhong, 2013, China [17] | Retrospective | NSCLC, SCLC | Combined | 60 | 56.5 for all | 68/52 for all | III, IV for all | 7 |
| TAI alone | 60 | |||||||
| Zhu, 2020, China [18] | RCT | NSCLC | Combined | 41 | 67.56 ±7.78 | 29/12 | III: 24 IV: 17 | – |
| TAI alone | 41 | 68.08 ±7.43 | 30/11 | III: 21 IV: 20 |
Three hundred and seventy-seven patients underwent combined TAI and ISI treatment (combined group), and 397 patients underwent TAI alone (TAI alone group). The ISI was performed under CT guidance. The results of therapeutic endpoints are shown in Table 2.
Table 2
Characteristics of the treatments
| Study | Trans- arterial treatment | Group | CRR | TSR | DCR | 1-year survival rate | 2-year survival rate | OS |
|---|---|---|---|---|---|---|---|---|
| Guo [11] | TAI | Combined | Not given | 40.8% | Not given | Not given | Not given | 15.1 months |
| TAI alone | Not given | 22.3% | Not given | Not given | Not given | 10.1 months | ||
| He [12] | TAI | Combined | 48.0% | 84.0% | 94.0% | 90.7% | Not given | Not given |
| TAI alone | 0.0% | 45.1% | 78.4% | 64.6% | Not given | Not given | ||
| Li [13] | TAI | Combined | 60.0% | 86.7% | Not given | Not given | Not given | Not given |
| TAI alone | 0.0% | 53.3% | Not given | Not given | Not given | Not given | ||
| Li [14] | TAI | Combined | Not given | Not given | Not given | Not given | Not given | 22.8 months |
| TAI alone | Not given | Not given | Not given | Not given | Not given | 14.2 months | ||
| Lin [15] | TAI + E | Combined | 26.5% | 76.5% | 91.1% | Not given | Not given | Not given |
| TAI alone | 5.6% | 50.0% | 66.7% | Not given | Not given | Not given | ||
| Xing [16] | TAI | Combined | Not given | 82.5% | Not given | 82.5% | 63.2% | Not given |
| TAI alone | Not given | 46.7% | Not given | 33.3% | 6.7% | Not given | ||
| Zhong [17] | TAI | Combined | 50.0% | 86.7% | 96.7% | Not given | Not given | Not given |
| TAI alone | 23.3% | 46.7% | 60.0% | Not given | Not given | Not given | ||
| Zhu [18] | TAI | Combined | 39.0% | 82.9% | 95.1% | 87.8% | 68.3% | Not given |
| TAI alone | 22.0% | 61.0% | 90.2% | 73.2% | 46.3% | Not given |
Quality evaluation
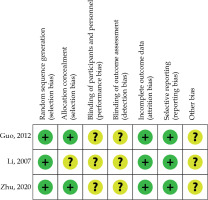
Figure 2 shows the bias risk of RCTs. All RCTs had an unclear risk of performance, detection, reporting, and other bias [11,13,18]. One RCT also had an uncertain risk for selection bias [13]. NOS concerning retrospective investigations ranged from 7 to 8 (Table 1).
Complete response rate
Five studies provided the results of CRR [12,13,15,17,18]. The pooled result indicated that CRR was significantly increased within the combination cohort than in the TAI cohort (44.0% vs. 12.3%, p = 0.001; Figure 3A). The heterogeneity was significant (I2 = 62%).
Fig. 3
Pooled results of A) CRR, B) TSR, C) DCR, D) 1-year survival rate, E) 2-year survival rate F) OS duration, G) myelosuppression rate, and H) gastrointestinal reaction rate between the two groups

The sensitivity analysis indicated that the significant heterogeneity disappeared (I2 = 28%) after removing He et al. study [12]. However, the CRR was still significantly increased in the combination cohort rather than in the TAI cohort (42.7% vs. 16.4%, p = 0.004).
Treatment success rate
Seven studies provided the results of TSR [11-13,15-18]. The pooled result indicated that TSR was significantly higher in the combined group than that in the TAI alone group (71.1% vs. 41.6%, p < 0.00001; Figure 3B). The heterogeneity was not significant (I2 = 7%), and sensitivity analysis was not required.
Disease control rate
Four studies provided the results of DCR [12,15,17,18]. The pooled result indicated that DCR was significantly increased within the combination cohort than in the TAI cohort (94.6% vs. 72.9%, p < 0.00001; Figure 3C). The heterogeneity was not significant (I2 = 26%), and sensitivity analysis was not required.
1-year survival rate
Three studies provided the results of 1-year survival rate [12,16,18]. The pooled result indicated that 1-year survival rate was significantly increased within the combination cohort than solely within the TAI cohort (86.5% vs. 57.6%, p < 0.00001; Figure 3D). The heterogeneity was not significant (I2 = 29%), and sensitivity analysis was not required.
2-year survival rate
Two studies provided the results of 2-year survival rate [16,18]. The pooled result indicated that 2-year survival rate was increased within the combination cohort than in the TAI cohort without significance (65.3% vs. 25.6%, p = 0.08; Figure 3E). The heterogeneity was significant (I2 = 88%). However, there were only 2 studies for this endpoint. Therefore, sensitivity analysis could not be performed.
Overall survival
Two studies provided the results of OS duration [11,14]. The pooled result indicated that OS duration was significantly longer within the combination cohort than in the TAI cohort (p = 0.0002; Figure 3F). The heterogeneity was significant (I2 = 98%). However, there were only 2 studies for this endpoint. Therefore, sensitivity analysis could not be performed.
Myelosuppression
Two studies provided the results of myelosuppression rate [12,15]. The pooled result indicated that myelosuppression rates were comparable between both the cohorts (50.0% vs. 64.4%, p = 0.29; Figure 3G). The heterogeneity was significant (I2 = 66.0%). However, there were only 2 studies for this endpoint. Therefore, sensitivity analysis could not be performed.
Gastrointestinal reaction
Two studies provided the results of gastrointestinal reaction rate [12,18]. The pooled result indicated that the gastrointestinal reaction rate was significantly higher in the TAI alone group than in the combined group (50.0% vs. 36.3%, p = 0.02; Figure 3H). The heterogeneity was not significant (I2 = 48%), and sensitivity analysis was not required.
Sub-group evaluations
The sub-group evaluations were conducted depending on different cancer types (Table 3). Five studies only included NSCLC [11,13-15,18], and 3 studies included both NSCLC and small-cell LC (SCLC) [12,16,17]. When focusing on the NSCLC alone, the CRR (p = 0.02), TSR (p < 0.0001), and DCR (p = 0.02) were significantly elevated in the combination cohort than in the TAI cohort. When focusing on the NSCLC and SCLC, the TSR (p < 0.0001), DCR (p < 0.0001), and one-year survival rates (p < 0.0001) were significantly increased in the combination cohort than in the TAI cohort. However, CRRs were comparable between the two groups (p = 0.16).
Table 3
Meta-analytic results based on the studies with different types of cancer
Publication bias
Egger tests showed no significant risk of publication bias on the endpoints of CRR (p = 0.744), TSR (p = 0.356), DCR (p = 0.451), and 1-year survival rate (p = 0.225). For the endpoints of two-year survival rate, OS duration, myelosuppression rate, gastrointestinal reactivity rate, and quantities of included studies were smaller than 3; therefore, Egger test could not be used, whereas funnel-plots did not show significant publication bias risks.
Discussion
The present meta-analysis provided a comprehensive evaluation of combined TAI and ISI therapy for patients with advanced LC. The clinical efficacy was mainly evaluated based on treatment response, long-term survival, and treatment-related toxicity.
For the patients with advanced LC, traditional systemic chemotherapy and radiation therapy should be initially considered [2]. However, some patients may be difficult to treat with standard chemotherapy and thoracic radiation therapy as a result of poor Eastern cooperative oncology group performance status (ECOG PS) (≥ 2), advanced age (≥ 70 years), severe hepatic failure, severe respiratory failure, refusal of traditional chemotherapy, or failure to treat with standard therapy [4-7]. Under this condition, local treatments including ISI and TAI are usually used for patients who are difficult to treat with standard therapy [4-7].
Therapeutic consequences are vital outcomes concerning oncology therapy investigations [22-25]. However, in previous studies, the clinical efficacy of TAI and ISI alone was limited, with the CRRs ranging between 0.0-2.5% and 12.5-23.0%, respectively [4,6,7,26]. In addition, TAI alone was usually limited by multiple feeding arteries of the tumor [4,6]. On the other hand, ISI could constantly release reduced energy gamma rays and maintain tumor areas irradiated [27]. However, the clinical efficacy of ISI can be further improved using adjuvant chemotherapy [5]. Therefore, many researchers combined ISI and TAI together to achieve a better treatment effect for advanced LC.
In the present study, the pooled CRRs indicated that ISI could significantly improve the clinical efficacy of TAI for advanced LC. Furthermore, the pooled CRR of combined treatment was 44.0% higher than in previous studies [4,6,7,26]. A previous meta-analysis found a pooled CRR of 21.5% after combined ISI with systematic chemotherapy [5], which was lower than in the present study. This finding can be attributed to the first-pass effect as a mechanistic path adopted by TAI [4]. Localized potentiation of chemical medication within the designated lesion region employing TAI could obtain 2-6× fold efficacy compared to conventional systematic chemotherapeutic options [28].
The significantly improved TSR and DCR were also observed in the combined group. However, CR could not be achieved by half of the treated patients, while the pooled TSR and DCR of combined treatment could reach up to 71.1% and 94.6%, respectively. Furthermore, the low heterogeneity of these endpoints also improved the stability of pooled results. These findings indicate that combined treatment is superior to TAI alone in treatment response, while combined treatment can control the LC progression in most patients.
The survival function was assessed by the survival rates and OS duration in this meta-analysis. Previous studies reported the median OS duration for advanced LC of 9-16 months, with 28.0-31.0% one-year survival rates after TAI or ISI alone [4,6,7]. Our pooled one-year survival rate and OS duration were significantly superior within the combination cohort than within the TAI cohort. Furthermore, the 1-year survival rate after combined treatment reached 86.5%. These findings can be attributed to better TSR and DCR after combined treatment. However, the significant heterogeneity of OS duration caused the result of OS and should be further validated.
The pooled 2-year survival rates were not significantly different between the two groups, which may indicate that combined treatment has a limited effect on long-term cancer control. This phenomenon may be attributed to the fact that the activity of 125I seeds reduce along with the time flowed. However, high heterogeneity indicate unstable results. Further studies are still required for conclusive results.
Myelosuppression and gastrointestinal reaction were the most common treatment-related toxicity after chemotherapy. Our meta-analysis results indicated that ISI did not aggravate TAI-related toxicity. However, the significant heterogeneity of myelosuppression should be further validated.
Most chemotherapy and/or radiotherapy studies focused on NSCLC alone [22,23,27,28]. However, this meta-analysis included both NSCLC and SCLC. Therefore, we performed sub-group evaluation depending on the variable tumor models. The results indicated that cancer types did not influence the treatment effect of combined TAI and ISI. Although the CRRs were comparable between the two groups based on the sub-groups of NSCLC and SCLC. The significant heterogeneity (I2 = 84%) indicated that this result requires further validation.
The present study had some limitations. Firstly, some investigations were retrospective in nature, and were associated with a high-risk of bias. In addition, some articles did not provide the data regarding stage and age distribution [11,13,15-17], which further increased the risk of bias. Therefore, more comprehensive RCTs are required. Secondly, TAI is a minimally invasive treatment that establishes a route to supply the local and low-dose chemotherapy. In this meta-analysis, TAI protocols, including types of medicine, dose, and circles of treatment were not the same in the included studies. These findings may further increase the risk of bias. Thirdly, multiple LC types possibly added further selection bias within such dataset outcomes. We did not perform sub-group analysis based on different tumor stages because the included original studies did not report the results based on different tumor stages. Therefore, an individual patient data (IPD) meta-analysis is needed to provide a more comprehensive and detailed results.