Purpose
Prostate carcinoma is increasingly prevalent due to improved life expectancy and early diagnosis. The suspicion of development of a carcinoma due to elevation of prostate specific antigen (PSA) in blood tests allows diagnosing carcinomas in early stages, most of them organ-confined, that is, without extra-capsular extension, and without extension to lymph nodes or distant organs. This group of patients can be treated with surgery, radiation-associated or not, hormonal therapy, or even managed with active surveillance in early cases. Each treatment has its’ indications, but the choice of treatment depends on many factors, such as experience of each center, availability of a technique, age, life expectancy, and doctor’s and patient’s preferences. All being effective in short-term, a longer follow-up shows variations in biochemical control of the disease, which is an elevation of PSA indicating new tumor growth. Side effects are also different and influence the decision on treatment technique according to age and characteristics of each patient. Different options of a salvage treatment in the event of a relapse of a primary treatment also influence the decision.
According to the European Urology Association (EUA), the EORTC and the American Urology Association (AUA), and National Comprehensive Cancer Network (NCCN) [1], tumors are divided into three risk groups for a relapse: low-risk, when PSA is < 10 ng/ml, tumor located in the prostate T1-T2a, and histological classification with a Gleason score ≤ 6; intermediate-risk, means having an extensive intra-capsular tumor that is palpable or visible by image (T2b-c) or Gleason 7, or PSA between 10 and 20 ng/ml; high-risk, when the tumor ruptures the capsule (T3) or PSA is > 20 ng/ml, or Gleason score ranges between 8 and 10. There are several classifications with modifications. According to the Seattle classification and the Mount Sinai Hospital in New York, the presence of two intermediate factors is regarded as high-risk. The latter also considers a T2b-c high-risk, as does the D’Amico classification [2], widely used by urologists. There is a good deal of agreement with low-risk groups (although in Seattle T2b is listed as ‘low’). What is more difficult, is to classify intermediate-risk group, which is a mixed bag that complicates the analysis of comparative results between different centers [3].
Prostate brachytherapy (BT) with permanent radioactive seed implants has been applied for at least 40 years, having proven its efficacy in organ-confined cases. When it comes to high-risk cases, it can also be used but combined with external radiation therapy (EBRT). At intermediate-risk, its efficacy as an exclusive treatment is not clear. There are many publications with iodine-125 (125I) seeds, but not so many with long-term follow-up, and they often combine patients from different risk groups. As prostate cancer is slow growing, 5-year biochemical control results are good with various techniques. However, they worsen over time and it is important to know the long-term efficacy. In addition, there are different techniques with seeds, with prior planning or with real-time, and various seed construction systems, with variable implant dosimetry quality, including loose, linked, and intra-operatively built custom-linked seeds [4]. A good technique and optimized dosimetry are able to improve the results at five years, while comparing two groups of similar patients [5].
The purpose of this work was to present the experience of a single-center with a large volume of patients over 10 years, with a real-time technique, a customized seed construction system, and with implants of good quality of dosimetry. The use of high-dose-rate (HDR) in monotherapy or stereotactic body radiation therapy (SBRT) is currently increasing, and low-dose-rate (LDR) is used at a continually decreasing rate. The reason for this study was to investigate if the long-term results of LDR-BT are sufficient to continue applying this technique, to know the importance of using an optimal system, and to discover risk factors that require a change in approach.
Material and methods
In October 2007, the 125I seed technique with pre-planning and ligated seeds (RapidStrand™) was changed to a real-time technique with intra-operatively constructed seeds (QuickLink™, Bard Medical). From that moment on, all patients who underwent permanent BT implantation, either exclusively or in combination with EBRT, were prospectively registered. All patients signed informed consent. Between October 2007 and June 2016, 770 implants were performed. Those with a follow-up of less than two years (8 deaths, 40 lost to follow-up), 4 cases with previous EBRT in this area, 5 high-risk cases, and 7 patients with implants as salvage to relapse of previously irradiated cases, were excluded. Therefore, 706 patients were analyzed, 480 of low-risk and 226 of intermediate-risk, according to the EORTC and NCCN (2016) classification.
Regarding treatment, 568 cases (80.5%) were exclusive with seeds, prescribing a dose of 145 Gy to the prostate, and 138 (19.5%) received prior EBRT on prostate and seminal vesicles, using 3D technique with seven fields shaped up to a dose of 46 Gy plus a seed implant at a dose of 108 Gy two to four weeks later. Hormonal treatment with bicalutamide for four weeks and luteinizing hormone-releasing hormone (LH-RH) analog was administered in 19.2% of cases, some prescribed by their urologist for six months, others for three months to reduce volume of the prostate and be able to perform the implant in a more suitable way. In total, 15% of low-risk cases had androgen deprivation therapy (ADT), and 27% of intermediate-risk patients. Characteristics of the patients are shown in Table 1.
Table 1
Characteristics of the 706 patients
All the patients were evaluated by means of a prostate volumetry a few weeks before to know the size, shape, and feasibility of the implant, and to order the seeds with adequate activity. In some cases, multiparametric MRI was performed, but not systematically. BT implant was performed under spinal anesthesia. Ultrasound images were taken every 5 mm. Then, needles were inserted to cover periphery of the prostate, the image was recorded again, and the dosimetry was calculated. It was then decided whether it was necessary to add central needles. In the same operating room, rows of seeds and spacers were built using Bard QuickLink™ system, avoiding using loose seeds. Before concluding, the final dosimetry was evaluated to insert some seed in uncovered areas, so that good coverage and dosimetry were achieved in vast majority of cases.
Follow-up at one month and a CT scan were performed for subsequent dosimetry. PSA control started at three months, then every 4-6 months, up to 5 years, and then annually. Biochemical relapse was considered according to the Phoenix criteria, when PSA rises to nadir + 2 ng/ml. During the first two years, there were frequent cases of transient elevation; therefore, despite a criterion of relapse, no further investigations were required other than close monitoring of PSA every three months. To achieve a more homogeneous population, only patients with at least two years of follow-up were included in this study. Regarding toxicity, CTCAE v. 5.0 classification was applied [6].
For statistical analysis of data, SPSS-15 statistical program was used to determine biochemical relapse-free survival (BRFS) by means of Kaplan-Meyer actuarial method. Comparison between series was made with log-rank test, considering p < 0.05 as significant value.
Results
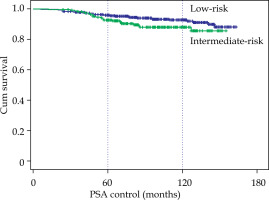
The median follow-up was 96 months, with a range between 24 and 163 months. BRFS at 5 and 10 years were calculated (Table 2), and for the 706 cases, it was 95% and 91.1%, respectively. For the 480 low-risk cases, BRFS was 95.7% and 92.7%, and for the 226 intermediate-risk cases, it was 92.7% and 88%, a significant difference (p < 0.05) compared with low-risk cases (Fig. 1). With EBRT + BT combined treatment, 5 low-risk cases (due to inadequate implants) were treated without relapses, and 133 intermediate-risk cases (59%) without significant differences, with the cases of exclusive BT.
Table 2
Biochemical relapse-free survival (BRFS) at 5 and 10 years
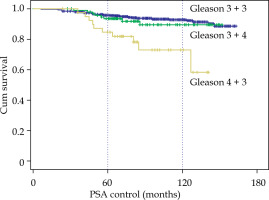
According to Gleason score, no differences between 3 + 3 and 3 + 4 were observed, but with Gleason 4 + 3, BRFS dropped to 84.7% and 72.8% at 5 and 10 years (p < 0.001), respectively (Fig. 2). In patients receiving ADT and in those not receiving ADT, G3 + 3 and G3 + 4 were similar; but in G4 + 3, in patients treated with hormones, BRFS at 10 years was 90.9% and in those without hormones, 66.8% (p < 0.01) (9 relapses in 29 cases). When studying the cases with EBRT + BT, a difference was also observed only for Gleason score 4 + 3, with BRFS at 10 years going from 75.5% to 60% in the 5 cases who received BT only.
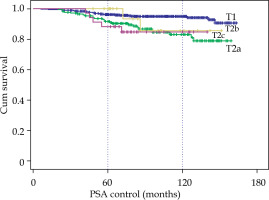
By clinical stage, the 10-year BRFS for T1c was 95% compared to 83% for T2a, 85.6% for T2b, and 84.7% for T2c. Moreover, a very significant difference (p < 0.001) between T1 and T2 (no difference between T2a, T2b and T2c) was observed (Fig. 3).
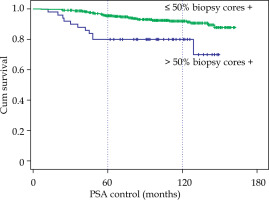
However, the initial PSA greater or lower than 10 ng/ml did not show differences with a 10-year BRFS of 91.6% with PSA < 10 vs. 88.9% with PSA between 10 and 20. The involvement of more than 50% biopsy cores did show a clear difference, 92% vs. 80% at 10 years (p < 0.001), and most relapses appeared in the first four years (Fig. 4).
Fig. 4
Biochemical relapse-free survival according to the number of positive biopsy cores, ≤ 50% or > 50%
Taking age into account, only 3 of 154 younger patients, up to 60 years of age relapsed, with a 10-year BRFS of 97.6% as compared with 89.4% in patients older than 60 years (p < 0.013).
Dosimetry at the end of the implant with exclusive BT achieved a V100 of 97.3 ±1.9% (range, 84.7-99.9%), and D90 of 169 ±8 Gy (range, 131-189 Gy) in the prostate. In cases with EBRT + BT, V100 was 97 ±2% (range, 85-100%) and D90 was 124 ±7 Gy (range, 102-137 Gy).
We reviewed 453 cases with PSA figures available at five years (excluding earlier failures or shorter follow-up), and the median PSA was 0.1 ng/ml (interquartile range, 0.04-0.2). Only one patient with PSA ≤ 0.2 suffered a biochemical recurrence.
Regarding toxicity, complications were recorded in 150 patients (21.2%): in the cases of exclusive BT (18.3%) and in those of EBRT + BT (33.3%). Urinary toxicity was observed as 18.7% (16.9% in BT alone vs. 26.1% in EBRT + BT), grade 2+ complications in 13.3%, and grade 3+ in 5.8%. Rectal or digestive toxicity was very low: 4% (2.5% in BT alone and 10.1% in EBRT + BT), grade 2+ complications in 1.6%, and grade 3+ in 0.1%. Table 3 describes urinary complications, and Table 4 shows digestive complications.
Table 3
Urinary complications
Table 4
Rectal complications
A comprehensive study investigating sexual function has not been carried out; it is difficult to assess in the long-term due to natural loss of function due to age.
Discussion
Radioactive seed brachytherapy of the prostate is an effective and widely used established treatment. In recent years, the number of implanted patients has been reduced due to greater access to robotic surgery as the first option for radical prostatectomy, and extensively used HDR-BT, available in more radiation oncology departments. PSA control results are good with all techniques in low-risk cases, due to slow growth of these carcinomas, which makes it possible to choose even active surveillance in very low-risk cases. In intermediate-risk cases, however, there are highly variable results, depending on the center and experience. It has been known for years that brachytherapy offers results that surpass EBRT or surgery [7], with recent meta-analysis confirming this theory [8]. Long-term results have been published, few with 10 years or more of follow-up, and a decrease in PSA control is always observed [9], achieving long-term PSA stability in 86% of cases treated with radioactive seeds [10]. Today, younger patients are treated thanks to early diagnosis by PSA screening. For this reason, it is essential to know the long-term results to offer all alternatives to patients. There are salvage treatments, but not all of them are effective, so the best primary treatment with a long expectation of control should be chosen, resulting with fewest possible complications.
In a well-known publication by Grimm et al. [11] comparing BRFS of different techniques, BT with radioactive seeds stands out as the one that offers the best long-term results at low-risk and alone, or in combination with EBRT at intermediate-risk. The results of the Seattle group [12, 13] at 10 years in low-risk group show 90% with seeds, and in the Mount Sinai Hospital of New York, 90% at 12 years [14]. Our study obtained 92.7% at 10 years, which indicates that it is undoubtedly a good treatment, even in young patients who are offered surgery more frequently.
In the intermediate-risk group, the Seattle group obtained 84% at 10 years with seeds, and 88% with combined EBRT + BT treatment. The Mount Sinai group reached 84% at 12 years in 973 patients [15]. In our center, we have achieved a 10-year BRFS of 88%, associating EBRT in 59% of the 226 intermediate-risk cases. The results of EBRT in hypofractionation studies with 20 fractions, such as CHHiP [16] or PROFIT [17], show that the results compared with standard EBRT are equal in intermediate-risk, so hypofractionation is considered recommended, with a 5-year BRFS ranging between 85% and 90.2%, but at 8 years, they drop to 74%. The NRG/RTOG 0126 study among 748 intermediate-risk patients treated with 79.2 Gy reported an 8-year control of 80% [18]. A Spanish multicenter RECAP study with 1,294 patients had a BRFS of 88.9% at 5 years, but decreased to 71.4% at 10 years [19]. All these indicate that long-term results worsen when using only EBRT with or without hormonal therapy; therefore, maintaining 88% control at 10 years in the intermediate-risk group of our study was highly significant. Two studies comparing seeds as boost versus exclusive external RT (ASCENDE-RT [20]) and with EBRT or surgery [21], clearly show the advantage of using seed BT.
In our study, we verified that there are risk factors that reduce control, especially Gleason score 4 + 3 and > 50% biopsy cores positive. The appearance of long-term biochemical relapses in these cases indicates a more aggressive disease and requires a different approach. It is necessary to change from exclusive BT to a triple treatment combined with EBRT of the prostate and seminal vesicles, BT boost with seeds, and ADT for 6 months [22, 23]. The use of hormonal therapy was not significant in our study, except for unfavorable cases. It must be taken into account that there are low-risk cases who received ADT for 3 months to reduce prostate volume. A recent meta-analysis recommends that approach in such as in our study unfavorable cases [24]. The 2018 NCCN recommendations already propose a new classification that divides intermediate-risk cases into favorable and unfavorable, and their management should be different [25, 26]. This group of patients will be the subject of more detailed analysis in another publication.
Age is an important factor when choosing primary treatment, since there is a tendency to recommend surgery as the first option in young patients, due to long life expectancy; although BT appears to offer at least similar results [27]. In our study, patients up to 60 years of age had better control than older patients (97.6% at 10 years vs. 89.4%), which makes the option of BT with radioactive seeds clearly recommended, allowing better preservation of erectile function [28]. Long-term BRFS rates of 96-98.9% have been published in men aged 55 or younger [29, 30].
Regarding toxicity, we found that the association of EBRT and seeds supposes a greater risk of urological (from 16.9% to 26.1%) and rectal complications (from 2.5% to 10.1%). Generally, the use of external RT increases the risk of digestive toxicity [31]. Even so, urological G3 toxicity occurs only in 5.8% and rectal G3 toxicity in one case, fewer than the data published in the ASCENDE-RT study (only 6% of toxicity was persistent) [32].
The technique used is important, since a real-time system and placement of fixed intra-operatively built custom-linked seeds allows for better dosimetry and lower doses to organs at risk. We achieve excellent dosimetry, with mean V100 of 97% and mean D90 of 169 Gy with exclusive BT, and 124 Gy with combined treatment. This can be one of the reasons why the results from multiple hospitals are not so favorable. A collection of 582 intermediate-risk cases treated with exclusive seed brachytherapy in eleven Italian hospitals obtained an 8-year BRFS rate of 78.4% [33], 10% lower than in our study. It is essential to use a good implant technique to recommend BT as the first option over EBRT or prostatectomy [5, 34].
Treatment of low- and intermediate-risk patients with two-session high-dose-rate BT or 5-session SBRT is promising, but we do not yet achieve 10-year results [35]. We are aware that biochemical relapses can appear in long-term, if a PSA level at 4-5 years below 0.2 ng/ml is not reached, what is considered a biochemical definition of cure [36]. Moreover, an analysis of 3,502 patients showed that LDR-BT led to lower nadir PSAs, with longer continued decay compared to SBRT and HDR-BT [37].
Low-dose-rate brachytherapy is in decline, but with the results obtained in our experience with the real-time technique and custom-linked seed system, the implantation of 125I seeds in a single-day still remains the most attractive option to achieve long-term control.
Conclusions
Brachytherapy with 125I seeds is a very effective long-term treatment for low- and intermediate-risk carcinoma of the prostate. In 706 patients, we obtained a 10-year BRFS of 92.7% in low-risk and 88% in intermediate-risk patients. In cases with Gleason score 4 + 3 or > 50% biopsy cores positive, there are more relapses, which coincides with a recommendation to consider them as an unfavorable intermediate group, and we recommend giving triple treatment with EBRT + BT with additional ADT for 6 months. The real-time technique with intra-operatively built custom-linked seeds allows implants with high quality dosimetry, and makes it possible to achieve excellent biochemical control, which is maintained in the long-term. Toxicity is limited in cases with combined treatment and very low with exclusive BT. Based on these results, BT with 125I seeds should be offered to selected patients as one of the main therapeutic options, especially to young patients.