Introduction
Coagulation factors are produced by the fetus, which could be detected as early as after 10 weeks of pregnancy [1-3]. The concentration of coagulation factors in the fetus increases gradually [4] and is lower in premature babies, compared to full-term newborns and older children [2, 3]. After delivery, the concentration of vitamin K-dependent coagulation factors (II, VII, IX, and X) and contact factors (XI, XII, prekallikrein, high molecular weight kininogen) are approximately 25-70% of the concentration in adults [3], while the concentrations of factors V, VIII, XIII, and fibrinogen are comparable to those found in adults [5, 6]. The immaturity of coagulation proteins at this age is compensated by low (approx. 50% of adults) concentration of inhibitors of the coagulation system (protein C, protein S, antithrombin, and heparin II cofactor) and reduced plasma fibrinolytic activity [2, 3, 7]. At the early stage of life, the coagulation system has a little of resources and compensatory capacity in response to external factors, resulting in an increased tendency for both thrombosis and bleeding [2, 3]. In most children, coagulation proteins reach normal levels at nearly 1 year of age [3, 6].
Fetal, perinatal thrombosis is a rare event mainly associated with risk factors (Table 1). The most common risk factor is the presence of vascular (especially central and umbilical) catheters [1, 3, 8, 9]. The most frequent localization of thrombosis is renal vein with or without an involvement of inferior vena cava (IVC), central nervous system veins, and portal or mesenteric vein [1, 2, 10]. Renal vein and IVC thrombosis in the perinatal period occur with an estimated frequency of 2.2 per 100,000 live births and have a diverse clinical manifestation [2, 3]. Due to the compression by the arteries, thrombosis is the most commonly observed in the left renal vein [11, 12]. The course of renal vein thrombosis can be acute, with the classic triad of symptoms, including hematuria, palpable abdominal resistance, and thrombocytopenia, which may be accompanied by anemia [3, 8, 13]. At times, however, the course of thrombosis is asymptomatic, with its consequences revealed in long follow-up [3, 13]. Particularly severe course is observed in bilateral renal vein thrombosis (25% of cases), which increases the risk of acute kidney injury that often requires renal replacement therapy and may result in child’s death [1-3]. Isolated IVC thrombosis without renal vein involvement is most often associated with the use of vascular catheters (inserted through the umbilical vein or veins of the lower extremities). Thrombosis in this location may be asymptomatic or manifest as swelling and bruising of the extremities and lower body [8].
Table 1
The aim of this study was to present a case of an infant with a massive abdominal vein thrombosis, who was admitted to our center with a preliminary diagnosis of nephrocalcinosis.
Case report
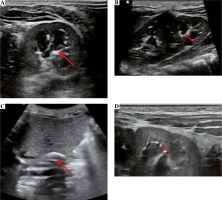
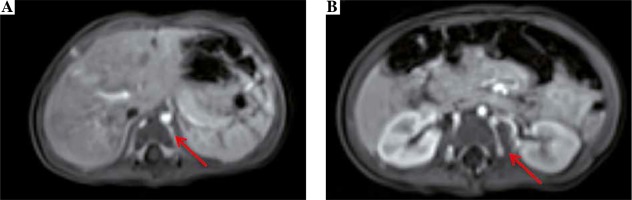
We present a case of a female infant born from second pregnancy (first pregnancy resulted in miscarriage in 20th week of gestation after abdominal injury), mother of Latin American descent, father of Polish origin. Negative family history of kidney diseases, calcium and phosphate homeostasis disorders, urolithiasis, systemic diseases, and thrombosis. The pregnancy was complicated by hypothyroidism, uterine myoma, urinary tract infections, and appendectomy at 16th week of gestation. Fetal ultrasound scan (US) performed repeatedly did not show any abnormalities, with normal image of the kidneys. The baby was born by caesarean section due to pre-labor rupture of the membranes, with a body weight of 3,570 γ and Apgar score of 10. The adaptation period was normal; the girl was vaccinated according to routine vaccination schedule and breastfed. At the age of 2 months, she was hospitalized in the city hospital due to bronchiolitis. The abdominal US showed kidneys 45 mm long with an abnormal image of the renal columns, with numerous hyperechogenic foci. Nephrocalcinosis was suspected and the infant was referred to our hospital. The girl was admitted at the age of 3 months in good general condition, no physical abnormalities, and arterial blood pressure of 77/36 mm Hg. Laboratory examination were as follows (reference ranges in brackets): WBC 8.06 × 103/µl (5.7-17), RBC 4.09 × 106/µl (3.2-4.3), PLT 415 × 103/µl (250-550), CRP < 0.5 mg/dl (≤ 1.0), creatinine 0.2 mg/dl (0.2-0.4), urea 14.0 mg/dl (2.2-27.8), calcium 10.5 mg/dl (7.7-11.5), phosphorus 7.2 mg/dl (2.5-7.0), alkaline phosphatase 281 U/l (80-345), 25(OH)D 20.4 ng/ml (20-50), parathyroid hormone 7.7 pg/ml (12-95); urinary crystallization ratio: calcium : creatinine ratio 0.43 (< 0.81), phosphorus : creatinine ratio 0.85 (0.3-1.2), magnesium : creatinine ratio 0.55 (> 0.1), magnesium : calcium ratio 1.29 (0.8-1.3). The results of urinalysis were within the normal range. In the ultrasound scan, numerous hyperechogenic foci in both kidneys were observed and nephrocalcinosis was suspected. Vitamin D3 supplementation was discontinued. After 3 months, serum phosphorus normalized (6.0 mg/dl), and 25(OH)D level was slightly below normal range (19.8 ng/ml). US showed right kidney 54 mm long, left kidney 62 mm long, linear calcifications along the pyramids visible in both kidneys, more lesions in the left kidney (Figs. 1A, 1B, 1D). Doppler scan showed lack of visible blood flow from the renal veins to IVC, with compensatory flow from the renal veins to the paravertebral plexuses; IVC obliteration, with a massive calcification in the hepatic section (Fig. 1C); minor linear calcifications in the liver caudate lobe at the point of contact with IVC. The connection between IVC and common iliac veins were not visible. The image suggested the history of extensive venous thrombosis. Magnetic resonance (MR) revealed no contrast flow in the iliac veins and IVC, compensatory venous outflow from the lower limbs and from the kidneys to the paravertebral plexuses (Fig. 2); in IVC, a segmental dilatation of 11 × 7 mm over a length of 23 mm spatially coincident with calcification described in the ultrasound scan. The results of coagulation system were as follows: INR 1.01 (0.9-1.2), kaolin-cephalin clotting time 34.62 s (28-40), fibrinogen 1.77 g/l (1.80-3.50), homocysteine 6.92 µmol/l (3.70-13.90), antithrombin III 96.3% (75-125), protein C 61% (50-125), protein S 67.36% (50-125), D-dimers 6893.06 µg/l (0-550). Leiden factor V mutation and c.20210G > A prothrombin gene mutation were excluded. Due to the increased concentration of D-dimers and obliterated IVC, low-molecular-weight heparin was administered at the therapeutic dose (nadroparin, 100 IU antiXa/kg once daily, subcutaneously). Anticoagulation was continued for 4 months. After a month of anticoagulation, D-dimer levels came back to normal (433.54 µg/l). Ultrasound scan performed at the age of 10 months showed: right kidney 60 mm long, left kidney 68 mm long, image of the venous system as in the previous study. The girl is currently 2 years old, develops according to age, and is under the care of a nephrology and hematology clinic. Obliterated venous vessels did not recanalize.
Discussion
Calcifications within the renal parenchyma (nephrocalcinosis) can include both the core and medulla (Table 2).Calcifications within the renal medulla are most often associated with metabolic disorders and indicate the need for extensive metabolic and genetic diagnostics. On the other hand, calcifications involving renal cortex indicate a history of necrosis (e.g., as a result of perinatal hypoxia or renal thrombosis) [10, 14, 15].
Table 2
Causes of calcifications within the renal parenchyma (nephrocalcinosis) according to [10] and [15] in own modification
In the described patient, no metabolic disorders that could increase the risk of precipitation of calcium salts in the renal parenchyma were found. As vitamin D hypersensitivity was suspected, which is usually associated with reduced activity of vitamin D inactivating 24-hydroxylase (mutations in the CYP24A1 gene), vitamin D supplementation was temporarily discontinued [15]. In the ultrasound scan, kidney lesions were not typical for any metabolic cause of nephrocalcinosis. Despite medullar location of the calcifications, thrombosis was suspected as the cause of observed lesions. Thrombosis was confirmed in the subsequent US and MRI. Although imaging studies did not clearly show the presence of thrombi in the renal veins, kidney lesions suggested a history of thrombotic process. It is difficult to clearly determine when massive thrombosis occurred. The presence of calcifications both in the kidneys and in the IVC lumen indicated that the event was distant in time; calcifications appear approximately 6 weeks after a thrombotic event [10]. Well-developed collateral circulation also supported the hypothesis on fetal thrombosis. On the other hand, high concentration of D-dimers found at six months of age could suggest that the thrombotic process was still active at that time. After a month of anticoagulation, D-dimer levels normalized. The described patient did not have the classic risk factors for thrombosis; the girl was born on time, there was no perinatal hypoxia, and no vessels were catheterized. No cause of congenital thrombophilia was found in the performed tests. The only detectable moment of possible fluctuations of fetal blood volume was acute appendicitis diagnosed in the mother at 16th week of gestation. However, this condition cannot be clearly linked to a history of thrombosis in the fetus.
In rare cases, venous and arterial thrombosis may occur in the fetal period [7, 16-21], and reports of in utero venous thrombosis diagnosed in a patient with renal calcifications are available in the literature [14, 16]. Belgian authors described the case of renal vein and IVC thrombosis diagnosed in utero. Comparably to the girl discussed here, the thrombosis resulted in calcifications in the renal parenchyma, with development of collateral circulation bypassing the obstructed IVC fragment [16]. On the other hand, massive IVC thrombosis in utero can lead to fetal hypoxia, capillary damage, and protein loss into the interstitial space, resulting in non-immune fetal edema [20], kidney failure [11], and death [22, 23]. Underdevelopment or malformations of IVC are found in approx. 0.3% of people. It is believed that intrauterine or perinatal venous thrombosis may be responsible for some of the cases of IVC agenesis [21, 24, 25]. In our patient, the thrombotic process led to complete obliteration of IVC and iliac veins. The case reported here and a few literature reports [14, 16] suggest that even a massive venous thrombosis in the prenatal period may remain completely asymptomatic for a long time. Diagnosis of IVC and renal veins thrombosis in the prenatal period is difficult. Such features as large hyperechogenic kidneys, increased echogenicity in the inter-lobe veins, thrombus visible in the IVC, increased resistance index, or reversal of renal artery flow in Doppler scan may suggest renal vein thrombosis [11]. Diagnosis of venous thrombosis is also possible in fetal MR imaging [21].
The decision of anticoagulant therapy administration in the case of neonatal or infant venous thrombosis is made individually [1, 2]. It is suggested that a transfontanellar ultrasound should be performed prior to the treatment to exclude active bleeding [10]. In most cases, the American Society of Hematology recommends unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) administration for 6 weeks to 3 months [1-3, 10]. In bilateral renal vein thrombosis resulting in acute kidney injury, the use of recombined tissue plasminogen activator (rtPA) is suggested, despite possible serious adverse effects of such a treatment [1, 3, 10]. Dosage and administration schedule for rtPA are available in the literature, and contraindications include recent surgery, intracranial bleeding or other active bleeding, convulsions, thrombocytopenia (< 100 × 103/µl), or hypofibrinogenemia (< 1 g/l) [3, 10, 13]. There are single case reports of successful local catheter-directed thrombolysis [26] or surgical thrombectomy in a newborn [9, 12]. In the discussed case, due to stable clinical course and difficulty in determination of the time of thrombosis, it was decided to administer low-molecular-weight heparin. The treatment was well tolerated by the girl and no progression was observed.
Thrombosis of IVC and its branches may be associated with serious distant consequences [8]. Renal vein thrombosis may result in kidney cirrhosis (approx. 70% of cases) and hypertension (approx. 20% of cases). Adverse consequences for the kidney can occur regardless of the treatment [1-3]. In the case of bilateral thrombosis, the patient is at risk of developing chronic kidney disease (CKD) with all its consequences. In patients with CKD, post-thrombotic occlusion of IVC or iliac veins may be of great difficulty, when placing vascular catheters for hemodialysis or kidney transplantation [10]. IVC obstruction poses a risk of impaired blood outflow from the lower body and distant complications, including post-thrombotic syndrome (erythema, edema, varicose veins, distal vein thrombosis) [9, 21, 27]. There is no doubt that children who underwent a perinatal thrombosis involving IVC and renal veins require regular follow-up by a nephrologist, with an assessment of renal shape and size, renal function, microalbuminuria, and blood pressure. Moreover, patients after IVC thrombosis require accurate and periodic clinical evaluation for post-thrombotic syndrome of the lower extremities [3].