Purpose
Currently, the standard treatment for ductal breast carcinoma in situ (DCIS) of less than 2-3 cm is conventional breast-conserving treatment (BCT) with external radiotherapy of the whole breast (whole breast irradiation – WBI). BCT reduces the risk of local ipsilateral breast tumor recurrence (IBTR) by approximately 50% compared to results with exclusive breast-conserving surgery (BCS): 8.9% recurrence rate at 15 years for BCT compared to 19.4% for exclusive BCS [1]. The benefit of BCT in local control is confirmed when analyzing different subgroups (age, grade, type, and clinical presentation), but has no influence on general or specific survival [2]. Local control mediated by BCT is influenced by several factors, such as resection margins, age, grade, presence of necrosis, and tumor multifocality. Of these, resection margins have the largest influence, providing almost 70% reduction in the risk of recurrence, with free margins of at least 2 mm [3,4]. Another factor to consider with BCT in DCIS is tumor multifocality, since it predicts the risk of residual disease after BCS [5].
Although in DCIS, the protective effect of adjuvant WBI after BCS is sufficiently documented [6,7], its systematic administration is still controversial, because the observed benefit is not uniform, does not improve survival, and involves administration of moderately high doses of radiation to large volumes of healthy tissue (lung, heart, and the remaining healthy breast without risk). Therefore, WBI in the conservative management of DCIS is considered an over-treatment in many cases [8,9,10]. Another limitation of conventional BCT is the duration of WBI (5 weeks), which can pose a logistic challenge for patients.
In selected patients with early breast cancer, accelerated partial breast irradiation (APBI) has become a standard post-operative treatment, since it is an alternative to standard BCT and has similar outcomes, while significantly reducing the duration of treatment and radiation exposure to organs at risk (OARs). A number of single-institution, balloon-based brachytherapy studies [11,12,13,14,15,16] confirmed that this treatment modality achieves adequate IBRT rates, good clinical, and cosmetic outcomes. Consequently, ASTRO guidelines include DCIS patients (≥ 50 years, screen-detected, low- to intermediate-risk, size ≤ 25 mm, and resected margins ≥ 3 mm) as suitable candidates for APBI [17].
To reduce the impact of over-treatment, and to overcome some of disadvantages of conventional BCT (radiation in healthy tissues and logistic problems associated with the duration of WBI), our institution launched a minimally invasive tumor bed implant (MITBI) program in 2008 to administer peri-operative high-dose-rate brachytherapy (PHDRBT).
This program uses an initial classification of the risk of recurrence based on definitive pathological data (margins/multifocality) to adapt the volume and intensity of adjuvant radiotherapy to the risk of each patient. Thus, in low-risk patients, accelerated minimal breast irradiation (AMBI) will be administer by exclusive PHDRBT, and in high-risk patients, early boost of the tumor bed (anticipated PHDRBT boost [A-PHDRBT boost]) will be performed in combination with WBI. The endpoint of this study was to evaluate the safety, efficacy, cosmetic results, and toxicity of the MITBI and PHDRBT (AMBI/A-PHDRBT-boost) program for DCIS.
Material and methods
Objectives of the MITBI and PHDRBT program (AMBI/A-PHDRBT-boost)
Primary objectives:
To optimize the recognition of radiation target by intraoperatively distinguishing the tumor bed within the surgical bed.
To perform a MITBI, rather than implanting the entire surgical bed.
To classify the risk of a relapse according to the status of margins and signs of tumor multifocality in the definitive study of a surgical specimen.
Secondary objectives:
To optimize the use of adjuvant radiation by performing accelerated minimal breast irradiation (AMBI) as an exclusive treatment in low-risk patients, or as early boost to the tumor bed (HDR anticipated boost) prior to hypofractionated external radiation therapy in high-risk patients.
To benefit the technical performance of multicatheter interstitial brachytherapy (stability of the applicator in the target and high dosimetric quality derived from a 3D planning by image-guided brachytherapy) that are especially attractive for treating small volume tumors.
To reduce radiation exposure of healthy tissue (lung, heart, and breast), decrease the risk of long-term toxicity, and improve cosmetic results in low-risk patients.
To improve the logistics of treatment, especially in low-risk patients treated with AMBI, by significantly decreasing the duration of locoregional treatment to 11 days (from surgery) compared to 60-70 days required for conventional BCT.
Inclusion criteria for MITBI and AMBI
Patients older than 40, with a vacuum-assisted biopsy diagnosis of DCIS that was less than 3 cm in size and confirmed by mammogram, ultrasound, and/or magnetic resonance imaging (MRI) as well as by surgeon’s criteria to be unifocal, were included in the study. The institutional board approved prospective recruitment for the study. All patients signed a consent form.
MITBI procedure
Usually, lumpectomy is performed through skin over the tumor. Once the lumpectomy is performed, the tumor bed must be recognized within the surgical bed. Tumor bed is the target of implant and corresponds to the projection zone of tumor on both cavity walls (marked with four titanium clips apart), surrounded with 1-2 cm (Figure 1C, D). The definition of the tumor bed is determined on the base of topographical information in pre-operative breast radiology imaging, information from clinical surgeon, and examination of surgical specimen.
Fig. 1
Minimally invasive tumor breast implant (MITBI). A) Tumor on surgical specimen, B) measurement tumor to margin, C) two of four titanium clips marking the projection of the tumor on superior wall of the surgical bed, D) tumor bed (yellow line) inside surgical bed (orange point line). Tumor bed include clips’ zone and 1-2 cm surrounding of breast tissue encompassed by the catheters
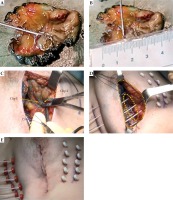
In the present study, the surgical clip zone and a margin of 1-2 cm was completely encompassed by the implant. Implantation was performed intraoperatively by freehand technique with sharp needles (Nucletron, Elekta AB, Stockholm, Sweden), and in all cases, the needles were placed perpendicularly to the axis to avoid geometrical disturbances and to promote wound-closure (Figure 1D). The implant extended from the depth to the surface and from the inside to the outside; the intraplane catheters were placed 1-1.5 cm apart, following Paris system recommendations. All patients received prophylactic oral antibiotics.
Regarding margin status on final pathology after surgery, reoperation indication was established by tumor on ink or close margin (less than 2 mm) on G3 histology.
MITBI-AMBI protocol treatment
Computed tomography planning was performed 48 hours after surgery and MITBI. Clinical target volume (CTV) was contoured from the recognition of clip zone and surrounding 1-2 cm encompassed by the catheters.
Based on definitive pathological results, patients with pure DCIS, tumor size < 3 cm, and margins > 2 mm with no signs of microscopic multifocality were classified as low- risk patients, and received 34 Gy of AMBI delivered in 10 fractions, 6 hours apart (3.4 Gy/fraction), over 5 consecutive days. Patients implanted with positive or very close margins not suitable for additional wide excision, or those with pathological microscopic multifocality classified as high-risk patients, received A-PHDRBT-boost (13.6 Gy in 4 fractions, 6 hours apart; 3.4 Gy/fraction over 2 consecutive days), followed by 39.9 Gy of hypofractionated whole breast irradiation in 15 fractions (START B), over 3 weeks after brachytherapy procedure.
The dosimetric parameters were defined as: CTV D90 coverage > 90%, dose homogeneity index (DHI) > 0.7, and the skin D10 < 70%. New GEC-ESTRO interstitial multicatheter APBI recommendations [18] were calculated retrospectively and analyzed. Patients with positive estrogen receptors received a hormonal treatment.
Follow-up and clinical outcomes
Follow-up included clinical examination by breast surgeons and radiation oncologists at 1 month after the brachytherapy procedure and every 6 months thereafter for 5 years. Mammogram and breast ultrasound were done annually. Ipsilateral breast tumor recurrence was classified as true failure (TF) or elsewhere failure (EF), according to clinical site in relation to the initial tumor bed. TF was defined as a recurrence within or immediately adjacent to the primary tumor site or irradiated area. EF was classified as an ipsilateral breast recurrence several centimeters from the primary site or in other breast quadrant. Local control was established when no IBRT was documented. Regional and distant metastasis were also recorded.
Early and late complications, acute and late toxicity, radiation-related effects, cosmetic evaluation, and radiologic findings
All early or late complications were recorded in an electronic chart. Acute and late toxicities were graded according to the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer (RTOG/EORTC) radiation morbidity scoring scheme [19]. Radiation-related effects were categorized as: breast symmetry (symmetry, acceptable difference, obvious difference, or marked difference), breast retraction (not visible, slightly visible, obvious, or marked), hyperpigmentation (not visible, slightly visible, obvious, or marked), catheter puncture marks (not visible, slightly visible, obvious, or marked), skin telangiectasia (not visible, slightly visible, obvious, or marked), and skin-subcutaneous atrophy or fibrosis (absence, light, moderated, or severe). Cosmetic evaluation was based on Wazer’s criteria: excellent, good, fair, or poor [20]. The presence of oil-cyst, fat necrosis, and symptomatic fat necrosis were assessed by a mammogram, ultrasound, and physical examination during follow-up. Data were collected and recorded at every clinical visit.
Statistical analysis
Univariate analysis of different clinical and treatment quantitative variables included its central tendency (percentages, mean, or median) or dispersion (range). Discrete variables were compared using Fisher’s exact test method and χ2 test, with p < 0.05 considered as statistically significant. Continuous variables were compared using Wilcoxon-Mann-Whitney U test (p < 0.05 statistical significance). Kaplan-Meier method was applied to calculate survival results from the date of surgery to the last follow-up visit. All the statistical analysis was performed with IBM SPSS Statistics 20 (IBM Corp., USA).
Results
Between November 2008 and October 2016, 44 patients were initially evaluated in the program as candidates for an implant and AMBI. One patient refused to participate, and 2 patients were excluded intraoperatively due to anatomical limitations (surgical cavity located very eccentrically in the inner superior quadrant, with very thin breast tissue). Among the 41 patients implanted, 4 were further excluded after definitive pathology reporting infiltrating carcinoma. Moreover, one patient refused reimplantation during reoperation; this patient was excluded from the analysis. In total, 36 patients consisted a cohort of the study. Pre-operative patient characteristics are described in Table 1.
Table 1
Pre-operative patients’ characteristics according to treatment protocol
MITBI-AMBI protocol treatment
Definitive pathological findings showed that 24 patients (67%) met the criteria for AMBI, and 12 patients (33%) for A-PHDRBT-boost. Reasons for anticipated boost were close margins in 4 patients (33%), multifocality in 6 patients (50%), and both adverse pathological features in 2 patients (16%). Table 2 shows definitive pathological and surgical characteristics. Median time from an implant to brachytherapy was 6 days (range, 2-9 days).
Table 2
Definitive, pathological, and surgical characteristics
Reoperation due to margin status
Six patients (16.6%) underwent re-excision for positive margins or presence of G3 DCIS less than 2 mm from the margin. Among these, 4 patients (66%) had a G3 DCIS. During the re-excision, the implant was replaced in 6 patients, 4 (66%) underwent AMBI, and 2 (33%) a boost. Details are shown in Table 2.
Reoperation due to bleeding
Three patients (8%) presented complications related to bleeding. Two were resolved with compressive measures, and one required surgical wound revision to confirm whether the cause of bleeding was related to placement of the catheter.
Dosimetric characteristics
Dosimetric parameters are described in Table 3. New GEC-ESTRO practical recommendations for multicatheter breast implant were included and reported. OARs constraints (skin, heart, lung, adjacent normal tissue, and rib), planning target volume (PTV), and implant parameters (DHI, dose nonuniformity ratio – DNR, and conformal index – COIN) were adequately respected and covered.
Table 3
Dosimetry
1 some constraint values are presented as absolute dose (Gy) and as relative percentage of prescription dose, following the GEC-ESTRO recommendations [18], 2 exclusively for lung and heart constraint values, we also calculated the EBRT dose received
Complication, toxicity, and radiological findings during follow-up
Early complications, acute toxicity, late toxicity, and radiation-related effects were evaluated in all the patients. The relevant data are described in Table 4. All patients in the AMBI group and 92% of patients in the boost group developed acute grade ≤ 2 toxicities. One patient in the boost group experienced a major acute RTOG grade 3 or higher toxicity, resulting in a bad cosmetic outcome. All late toxicities described in the study were grade ≤ 2. No major late grade 3, or higher RTOG skin or subcutaneous toxicities were documented in any of the groups. Moreover, no statistical differences related to toxicity were found between the groups.
Table 4
Early and late complications, and Radiation Therapy Oncology Group (RTOG) toxicity
Radiation-related effects, symmetry, retraction, hyperpigmentation, catheter puncture marks, skin telangiectasia, skin-subcutaneous atrophy, and fibrosis were assessed in all the patients. Although, these effects were not visible or were slightly noticeable in a percentage of the patients in both groups, worse symmetry (p = 0.021) and retraction (p = 0.001) were statistically significant in the A-PHDRBT-boost group compared to results in the AMBI group.
Radiological findings during follow-up and cosmetics outcomes
On radiological follow-up, oil cysts developed in 12 patients (33%), while radiologic fat necrosis developed in 11 (30%) cases, out of which only 1 (2.8%) was symptomatic and required surgical removal. No statistical differences were found between the groups regarding radiological findings.
Cosmetic outcomes in AMBI patients were excellent or good in 23 patients (95.8%), and fair or poor in one patient (4.2%). Cosmetic outcomes in A-PHDRBT-boost patients were excellent or good in 8 patients (67%), and fair in 4 patients (33%). The difference between the groups was not statistically significant (p = 0.034). Further information on all categories are described in Table 5.
Table 5
Radiation-related effects and cosmetic evaluation
Clinical outcomes
The median follow-up for all patients was 97 (range, 42-139) months. For the AMBI group, the median follow-up was 100 (range, 43-137) months, and for A-PHDRBT-boost patients, 89 (range, 42-119) months.
For all patients, the rate of local, elsewhere, locoregional, and distant control was 97.2%. For the AMBI group (n = 24), the rates of local, elsewhere, locoregional, contralateral, and distant control were 95.8%, 95.8%, 100%, 100%, and 100%, respectively. One true local recurrence and one elsewhere were reported in this group at 62.2 and 103 months of follow up, respectively; both cases were G3 DCIS. The cumulative 5-year incidence of IBTR in the AMBI group was 8.3% (2/24) (Figure 2). Furthermore, based on biopsy tumor grade (G1-2 vs. G3), the failure-free survival rate in the AMBI group tended towards statistically significant (p = 0.067) (Figure 3). Only one patient in the A-PHDRBT-boost group developed locoregional progression and systemic disease due to previous contralateral ductal infiltrating carcinoma diagnosed 16 years before MITBI for DCIS. All data are presented in Table 6.
Fig. 2
Ipsilateral breast tumor recurrence (IBTR) incidence in accelerated minimal breast irradiation (AMBI) group
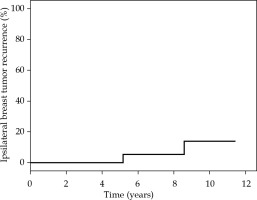
Fig. 3
Disease-free survival in the accelerated minimal breast irradiation (AMBI) group and biopsy grades
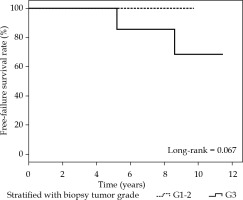
Table 6
Clinical outcomes by a grade
Discussion
The role of APBI has been explored in a variety of prospective studies (ELIOT, TARGIT, IMPORT LOW, Budapest experience, and GEC-ESTRO) [21,22,23,24,25]. In all of these studies, the tissue adjacent to surgical bed was protected using a safety margin of 1-2 cm. In the APBI reference studies, only the GEC-ESTRO study included 35 patients (5.5%) with DCIS in the APBI arm, without a control analysis by subgroup [25]. Institutional experiences with Mammosite showed no differences in the rates of IBTR between DCIS and the invasive suitable/cautionary risk group; this therapy also achieved good clinical outcomes, low acute and late toxicity, and acceptable cosmesis [26]. Despite the lack of strong bibliographic evidence, APBI has recently been included in the ASTRO guidelines as a suitable radiotherapy modality for the treatment of G1 and G2 DCIS of less than 2.5 cm, with free margins of more than 3 mm [17].
Among the different techniques used in the APBI reference studies of early breast cancer, multicatheter interstitial brachytherapy (MBI) offers the best recurrence rate to date, with a 5-year cumulative incidence of local recurrence of 1.4% [25]. Additionally, MBI offers very high performance in planning and dosimetry, with multiple dwell points throughout the implant, which allow scrupulous modulation, target shaping, and documentation of radiation dose to target and OARs.
Taking into account the strengths of the MBI technique (good recurrence rates and excellent dosimetric quality), we intended to use AMBI to enhance the effectiveness of adjuvant radiotherapy by reducing the volume treated to only the tumor bed with margin instead of the surgical bed with margin, as in the usual procedure with APBI techniques. Unlike post-operative interstitial multicatheter techniques, AMBI requires the tumor bed to be clearly distinguished from the surgical bed, so that irradiation can be delivered exclusively to the minimal breast tissue at risk after resection, with a maximal stability (interstitial implant), precise target conformation (multicatheter implant/3D dosimetry planning), and high-dose gradient. Finally, this procedure allows to achieve lower implant volumes compared to volumes treated using other devices or post-operative multicatheter APBI techniques.
In the present study, the median follow-up was 97 months (100 months in the AMBI group and 89 in the PHDRBT boost group). The incidence of IBTR in the AMBI group was 8.3% (2/24) and 0% (0/12) in the A-PHDRBT-boost group. The cumulative incidence of IBTR at 5 years for the entire study was 5.6% (2/36).
Although the median follow-up of our study was long, these results must be interpreted with caution due to limited number of patients. Nevertheless, the IBTR rate in our study is encouraging compared to 10-year IBTR rates of 15% that were reported for radiotherapy arms in reference WBI studies (NSABP B-17 and EORTC 10853), and when compared with recurrence rates exceeding 30% reported for exclusive lumpectomy arms [27,28]. Other authors have explored the efficacy of different APBI techniques to treat DCIS. Our 5-year rate of IBRT (8.3%) is comparable to IBRT rates reported with balloon-based implants (0.7% to 4.1%) [11,12,13,14,15,16] and with IOERT (11%) in patients considered suitable for APBI according ASTRO criteria [29].
In our study, G3 DCIS was associated with a high-rate of reoperation due to involved margins and with a worse proportion of IBTR compared to outcomes reported with standard treatments. Only two patients who developed IBTR in the AMBI group had high-grade tumors (G3); the IBRT rate for the whole group was 16% (2/12) and 25% (2/8) for the AMBI group (Table 6). Additionally, the percentage of reoperation for G3 tumors was high as 33% (4/12). For these reasons, in our institution, we decided that patients with a pre-operative diagnostic of G3 DCIS would not be included in the MITBI and PHDRBT program, but should be considered as candidates for whole breast irradiation.
It is important to note that, in patients with G1-2 tumors, the 5-year local control for the AMBI group was 100%. This finding shows the efficiency and selectivity of AMBI technique, in which the treated volume median V100 was 40 cc and the remaining median breast volume was 671 cc. These data suggest that the MITBI/AMBI combination for patients with low-risk G1-2 tumors significantly optimizes the use of adjuvant radiation by treating less than 10% (6.5%) of the breast, providing 100% of local control. In this special low-risk group of patients, the RTOG 9804 randomized clinical trial [7] with a median follow-up of 7.2 years reported a 6.7% IBTR rate without adjuvant radiotherapy, and a 0.9% IBTR rate for the whole breast radiotherapy arm. This result indicates that AMBI can offer local control, with IBTR rates similar to those of WBI, while minimizing the radiation dose to OARs and normal breast tissue.
This result (V100 less than 10% of breast volume) could also be clinically reflected in our study with cosmetic results and radiation doses to OARs. In AMBI group, 96% of patients achieved excellent/good cosmetic results compared to 67% rate in the PHDRBT boost group (p = 0.034). Furthermore, the higher BED achieved in the PHDRBT boost group regimen (anticipated boost and EBRT) could compromise the cosmesis. On the other hand, a significant reduction of unnecessary radiation exposure to OARs was documented: lung (median lung dose in AMBI = 1.13 Gy vs. median lung dose in A-PHDRBT-boost = 7.27 Gy, p = 0.0000062) and heart (median heart dose in AMBI = 0.93 Gy vs. median heart dose in A-PHDRBT-boost = 1.8 Gy, p = 0.01) in left-breast treatments.
Finally, the MITBI-PHDRBT program allows administration of adjuvant radiotherapy as early as possible once the definitive pathological report is known. This characteristic reduces the duration of treatment significantly (five days of PHDRBT treatment, 11 days from BCS until the end of treatment in the AMBI group) compared to 25-30 days (60-70 days from BCS until the end of WBI in conventional treatment) or 15 days (40-50 days from BCS until the end of WBI with current standard hypofractionated regimen).
In view of the improvements in efficacy, safety, and logistics, which are possible with the MITBI-PHDRBT and AMBI program, it is feasible to explore ultra-accelerated schedules in properly selected low-risk patients. Despite encouraging results with mature follow-up documented in low-risk patients treated with AMBI, the main limitation of this study was the small number of patients included. Therefore, all the interpretations must be taken with a caution.
Conclusions
The MITBI-PHDRBT program allow selection of patients with favorable prognoses (G1-2 DCIS with negative margins and absence of multifocality), for whom AMBI could be a good alternative with low recurrence rate, decrease of unnecessary radiation, treatment logistics improvement, and over-treatment reduction. Patients with G3 DCIS on pre-operative biopsy are at more risk of reoperation due to positive margins and worse local control; therefore the MITBI-PHDRBT for AMBI program is not recommended for these patients.



