Purpose
Endometrial carcinoma (EC) is the most common gynecological malignancy, with an incidence of 7% and 8,400 diagnoses and 2,516 deaths estimated in adult Italian women population in 2018 [1]. Total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO) represents the primary EC management, while adjuvant radiotherapy is routinely offered according to several adverse risk factors, including patient age (> 60-65 years), higher grade, higher stage, increasing tumor size, deep myometrium invasion (MI > 50%), presence of lymphovascular invasion (LVI), histology, and lymph node positivity [2]. Optimal management of early-stage EC remains unclear, requiring balancing the improvement of cancer-related survival, which is associated with new treatment techniques, and the impact of acute and treatment-related late toxicities that negatively affect the quality of life. Bearing in mind the long-term quality of life of these patients, several efforts have been made towards appropriate selection of patients, who do not need external beam radiotherapy (EBRT), but only vaginal cuff brachytherapy (VBT) as an adjuvant treatment. A firm conclusion has been drawn from a randomized PORTEC-2 trial, which reported similar rates of locoregional relapse in early-stage EC patients with intermediate-risk who receive exclusive VBT, when compared with pelvic EBRT [3]. Although VBT as monotherapy represents now the standard adjuvant treatment for early-stage EC, including intermediate- (IR) and high-intermediate-risk (HIR) disease, there is a large variability among fractionations or total dose or dose intensity delivery [2,4,5,6,7,8]. Despite this high variability of fractionations, VBT has been related to satisfied rate of local control, ranging from 90% to 100%, and low-rate of late vaginal toxicity, mainly G1-G2, with a range from 7.5% to 27.7% [6]. The most commonly employed fractionation consists of 21 Gy total dose, with 7 Gy each fraction, delivered once a week, as proposed by the PORTEC-2 trial [3]. Moreover, the experience of a Spanish group showed that it is possible to reduce the total treatment time without significant increase of late toxicities, using a daily fractionation of 6 Gy, three time a week [9].
The objective of our study was to analyze local control, survival outcomes, and toxicities in a consecutive series of patients with early-stage intermediate- and high-intermediate-risk EC, treated with exclusive HDR VBT to a total dose of 21 Gy, 7 Gy per fraction every other day.
Material and methods
Patient characteristics
The study group included 108 stage I EC patients who consecutively received adjuvant VBT between September 2008 and December 2018. The median age was 65 years (range, 35-86). Pre-surgery evaluation included patient’s history, physical examination, complete blood count, plated count, endometrial biopsy, endovaginal ultrasound, and/or total body computed tomography (CT) scan. All patients underwent surgery. The pathological stage was assigned according to FIGO classification from 2009.
All 108 patients presented endometrioid pathological subtype. Grade 1 disease was present in 14 patients (13%), grade 2 in 69 patients (64%), and 25 patients (23%) presented grade 3. MI > 50% was present in 57 patients (53%), while LVSI in 15 patients (14%). The overall stage distribution was as follows: IA in 50 of 108 (46%) patients and IB in 58 of 108 (54%). All patients showed histopathological features defined as intermediate-risk, stage IA G3 and stage IB (G1 and G2) with endometrioid type, according to FIGO definition from 2010 [10].
Patients were further re-allocated into intermediate-risk and intermediate-high-risk group according to ESMO-ESGO-ESTRO consensus conference as follows: 69 of 108 (64%) patients were classified as intermediate-risk endometrial cancer and 39 of 180 (36%) were categorized as high-intermediate-risk disease [2]. Patients characteristics are summarized in Table 1.
Table 1
Patient characteristics
Treatment
All patients underwent primary surgery consisting of total hysterectomy and bilateral salpingo-oophorectomy (TH/BSO) without node dissection in 41 patients (38%) and with bilateral pelvic lymph nodes dissection in 67 patients (62%).
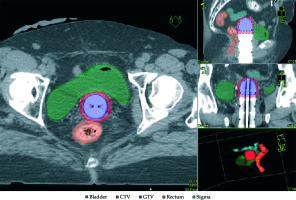
Vaginal brachytherapy was performed using an HDR unit with 192Ir source. CT-based treatment planning was applied for brachytherapy treatment by acquisition of 2.5 mm thicker slices, without contrast medium, using a personalized immobilization system, and repeating each time the same procedure. VBT was delivered via vaginal cylinders to the upper third of the vagina (the vaginal length treated was 3 cm). At the time of consultation, each patient was equipped with a vaginal cylinder, selected from custom-fabricated diameter sizes of 2.0, 2.5, and 3.0 cm. The largest possible diameter of vaginal cylinder was chosen for treatment to decrease vaginal mucosa dose, to improve depth dose, and to assure air gaps < 2 mm. In case of air pockets more than 2 mm, the diameter of cylinders was changed, and CT scan was repeated. Oncentra (version 4.5.3) treatment planning system was used for dosimetric calculations and treatment. Doses to the prescription depth, vaginal surface, rectal, and bladder point were calculated. The dose was prescribed at 5 mm distance from the applicator surface. Bladder and rectum were considered as organs at risk, with dose constraints as follows: for the bladder, a dose below 80% delivered to a volume of 5cc, and for the rectum, a dose below 75% delivered to a volume of 5cc and for each fraction. The dose was also calculated using the ICRU (International Commission on Radiation Units and Measurements) recommendations for bladder, rectum, and the maximum dose to vaginal mucosa. Figure 1 shows a representative dosimetrics of dose distribution. A total dose of 21 Gy was delivered in 3 fractions of 7 Gy, every other day, in one week (Monday, Wednesday, Friday) to all patients. To improve treatment compliance, all patients underwent topical therapy with hyaluronic acid after VBT and during the first year of follow-up.
Follow-up
The first follow-up evaluation was done at 6 weeks after the completion of VBT treatment. Thereafter, all the patients were evaluated every 4 months for the first 2 years, and every 6 months afterwards for at least 5 years, with clinical and pelvic examination and vaginal cytology. Chest radiography, and pelvic and abdominal ultrasound were carried out yearly, with additional CT and/or MRI as required. Subsequently, all patients started the annual follow-up, and the patients who did not attend a periodic clinical control in the last 2 years, were contacted by phone.
Toxicity assessment
Gastrointestinal, genitourinary, and vaginal toxicities were recorded using radiation therapy oncology group (RTOG) acute morbidity scoring criteria and late effects normal tissue task force – subjective, objective, management, analytic (LENT-SOMA) morbidity scale [11,12]. Late complications were assessed during follow-up, after 90 days after VBT completion.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software package version 13.0. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or date of the last follow-up. Cause-specific survival (CSS) was calculated from the date of diagnosis to the date of death from EC or date of the last follow-up. Disease-free survival (DFS) was calculated from the date of the end of radiotherapy course to the date of either distant metastases, or loco-regional recurrence or date of the last follow-up.
The Kaplan-Meier method was used to estimate the rates of OS, CSS, and DFS. We evaluated prognostic factors impact on OS and DFS. The association between survivals and categorical variables was assessed using log-rank test. Independent prognostic values of the factors that were statistically significant on univariate analysis were tested by multivariate Cox regression analysis model. The p value of less than 0.05 was considered as statistically significant.
Results
Patients characteristics
The median age at diagnosis was 65 years (range, 35-86). Details are presented in Table 1.
Survival outcomes
For the entire cohort, median follow-up was 44 months (range, 6-117 months). In total, 7 of 108 (6.5%) patients relapsed after a median time of 31 months (range, 5-56 months): 1 into vaginal vault and pelvic lymph nodes, 1 patient in pelvic lymph nodes, 1 to vaginal vault, pelvic lymph nodes, and distant site, and 4 cases developed metastatic disease.
According to stage and risk stratification, 5 patients were stage IA, 2 patients were stage IB, and 3 patients were identified with intermediate-risk and 4 patients high-intermediate-risk. Two of 7 patients died from a progressive disease, 4 patients were alive with endometrial cancer, and 1 patient is alive without endometrial cancer after second line of chemoradiotherapy.
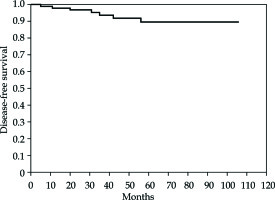
For all patients, the 5-year DFS was 89.5% (95% confidence interval [CI]: 93.018-103.793) (Figure 2). No difference in DFS was found when intermediate- vs. high-intermediate-risk groups were compared (p = NS).
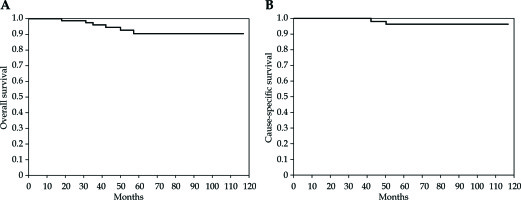
Overall, 102 (94%) patients are alive, of which 98 present no evidence of disease. Six (5.5%) patients died: 2 patients from EC and 4 patients from no cancer-related conditions. For all patients, the 5-year OS was 92.7% (95% CI: 104.39-115.337) and the 5-year CSS was 96.4% (95% CI: 111.012-117.917) (Figure 3). No difference in OS and CSS was found, when intermediate- and high-intermediate-risk groups were compared (p = NS).
Toxicity
Acute bladder toxicity G1-G2 was reported in 11 of 108 (10%) patients, vaginal toxicity G1-G2 in 6 of 108 (5.5%), and there were 3 cases (3%) of G1-G2 gastrointestinal toxicity observed.
Late bladder and gastrointestinal G1 toxicities were reported in 4 of 108 (4%) and 1 of 108 (1%) patients, respectively. Late vaginal toxicity (G1-G2) was recorded in 3 of 108 (3%) patients. No evidence of grade 3-4 bladder, vaginal, and gastrointestinal toxicities were noted.
Discussion
The role of exclusive VBT as a standard treatment in high-intermediate-risk EC patients has been recently confirmed by the long-term results of PORTEC-2 trial, in which, a dose of 21 Gy in 3 fractions of 7 Gy was delivered with one week interval between each fraction, and with the dose specified at 5 mm distance from the surface of cylinder [7]. Quite different fractionations and dose intensity (overall treatment time) schedule has been reported in literature, with local control ranging from 90% to 100% and with a rate of late vaginal toxicity, mainly G1-G2, ranging from 7.5% to 27.7% [6]. Other VBT schedules, which are used frequently include 5 Gy at 5 mm in 6 fractions, 5 Gy at 5 mm in 5 fractions, 7 Gy in 3 fractions at mucosa surface, and 5 Gy at 5 mm in 4 fractions [13]. Moreover, the American Brachytherapy Society guidelines also recommend not to exceed 2-3 fractions per week in order to reduce acute and late toxicities, namely vaginal toxicities.
As proposed by PORTEC trials, most frequent schedules adopted in other series of high-intermediate-risk EC patients for exclusive treatment are 3 fractions of 7 Gy, usually delivered with a frequency of 1-2 fractions per week and subsequent overall interval time ranging from 14 to 28 days [3,14,15,16,17,18,19,20,21,22,23,24,25].
In general, while using 7 Gy per fraction delivered with different overall treatment times, the 3-5 PFS survival was more than 90%, with a rather low-rate of acute and late toxicities (Table 2). Therefore, a lower number of VBT fractions is not detrimental regarding survival outcomes and toxicities incidence, while is better for the patient and requires less time for professionals. In almost all published studies, the overall treatment time was frequently longer than 2 weeks, ranging from 2 weeks to 4 weeks (Table 2). Rovirosa et al. recently showed no acute and late rectal and bladder toxicities in a short schedule of exclusive VBT, namely 5 Gy in 4 fractions daily [26]. With regard to vaginal toxicity, they recorded no G3-G4 acute and late toxicities, and only G1-G2 acute and late toxicities of 6.6% and 20%, respectively, that were within the range reported in literature. The same authors have subsequently published similar results on outcome survivals and toxicities with three daily fractions of 6 Gy, demonstrating that late complications are not more frequent when treatment is administered in less than 4 days, compared to more than 1-2 weeks or more prolonged time [9,27]. Results using short schedules of exclusive BT have been published by other several authors, showing similar conclusions to those of Rovirosa et al. regarding local control and acute or late complications. One hundred and twenty-two patients were treated with intravaginal HDR brachytherapy schedule, 5 Gy per fraction over five consecutive days, and recorded no late G3-G4 urinary, rectal, and small bowel G3 toxicities, and only 1 (0.8%) G3 late vaginal toxicity. Almost all the recorded toxicities were G1-G2 and, in particular, the authors showed late vaginal toxicities G1 and G2 of 6.9% and 1.7%, respectively [28]. Comparable results in terms of vaginal toxicities were published by Eiriksson et al., with a total dose of 35 Gy administered in up to 5 daily fractions for 1 week [29]. The authors recorded acute vaginal toxicities ranging from 1.3% to 6.5%, which were all treated and resolved during follow-up, with no clinically significant late complications.
Table 2
Published series on endometrial cancer patients treated with 7 Gy × 3 fractionated HDR brachytherapy schedule
| Authors | Number of pts | Median follow-up (months) | Stage | Fractionation | Fr/week | Local relapse rate (%) | Distant metastasis rate (%) | 3-5 yrs survival DFS/PFS% OS% | Acute toxicity G1-G2 % | Late toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| Fannings et al. [14] | 60 | 36 (12-65) | IB-IIIA | 7 Gy × 3 | Not specified | None | 1.6 | NR | Dysuria 3 Proctitis 2 Diarrhea 2 | Minimal vaginal stenosis |
| Weiss et al. [15] | 122 | 48 (3-93) | IA-II | 7 Gy × 3 | Not specified | 5.7 | 1.6 | 74-94 NR | Dysuria 10.6 | None |
| Chadha et al. [16] | 124 | 30 (7-91) | IB-IC | 7 Gy × 3 | Over 4 weeks | 0 | 2.4 | 87 93 | None | 2 pts vaginal stenosis |
| Horowitz et al. [17] | 164 | 65 (6-142) | IB-II | 7 Gy × 3 | Over 2 weeks | Vaginal 1.2 Pelvic 1.2 | 6 | 90 87 | 2 vaginal discharge 1 severe bladder infection | None |
| Alektiar et al. [18] | 382 | 48 | IB-IIB | 7 Gy × 3 | Three fractions in 2-week intervals | Vaginal 2 Pelvic 3 | 3.9 | 97 93 | 3 pts with G3 toxicities | 1 vaginal necrosis |
| Solhejem et al. [19] | 100 | 23 (2-62) | I-III | 7 Gy × 3 | Weekly | None | None | 93.3 97.9 | Dysuria 9 Diarrhea 9 Vaginal mucositis 17 | Vaginal atrophy, stricture, 16% |
| Nout et al. [3] | 213 | 45 (18-78) | I-IIA | 7 Gy × 3 | Weekly | Vaginal 1.4 Pelvic 3.7 | 7.5 | 82.7 84.8 | GI baseline level | GI G3, 1% G3 vaginal atrophy, 2% |
| Landrum et al. [20] | 23 | 44 (11-58) | IB-IIB | 7 Gy × 3 (CHT) | Every 72 h | Vaginal 4.3 Pelvic 8.6 | 8.6 | 87% NR | None | 2 pts dysuria 3 pts dyspareunia/stenosis |
| Perrucci et al. [21] | 157 | 83 (38-213) | I-II | 7 Gy × 3 | Weekly | Vaginal 1 Pelvic 8 | 1.2 | 93.6 96.5 | Vaginal 16 Bladder 5 Diarrhea 1 | Vaginal G1-G2, 55.4% Telangiectasia G1-G2, 40.8% Atrophy/ fibrosis, 14.6% |
| Laliscia et al. [22] | 126 | NR | IA-IB | 7 Gy × 3 | Weekly | Vaginal 3.9 Pelvic 2.4 Both 1.6 | 1.6 | 88 93 | Vaginal discharge 14.3 Dyspareunia 5.5 | Vaginal fibrosis, 11.1% Telangiectasia, 5.5% |
| Diavolitis et al. [23] | 169 | 103 (1-330) | IB | 7 Gy × 3 5.5 Gy × 4 | Not specified | Vaginal 1 Pelvis 2.3 | 4.4 | 94.4 NR | NR | NR |
| Cisek et al. [24] | 108 | 48.74 ±20.15 | IA-IB | 7/5 Gy × 3/4 | Weekly | Vaginal 2 | 2 | 96 93 | NR | NR |
| Dohopolsky et al. [25] | 297 | 52 (32-72) | IA-IB | 7 Gy × 3 | Not specified | Vaginal 1 Pelvic 2 | 5 | NR 91.8 | 2 vaginal dehiscence | 1 vaginal necrosis 1 vaginal dehiscence |
| Our series | 108 | 44 (6-117) | IA-IB | 7 Gy × 3 | 3 fractions/ one week | Vaginal 1.8 Pelvic 2.7 | 3.7 | 89.5 92.7 | Bladder G1-G2, 10 Vaginal G1-G2, 5.5 Gastrointestinal 3 | Bladder G1, 4% Vaginal G1-G2, 3% Gastrointestinal G1, 1% |
Our experience with 108 intermediate- and high-intermediate-risk patients, consecutively treated with one-week short VBT schedule as an exclusive adjuvant post-operative treatment, showed a 5-year RFS and CSS of 93.5% and 95.4%, respectively. In particular, we recorded only two vaginal relapses, while most of relapses occurred at pelvic and/or metastatic sites. With regards to the stage and risk factors, our series of patients was rather homogenous, being only stage I EC with intermediate- or high-intermediate-risk factors. We were not able to find any statistical correlation between relapse rate and each risk factors, mainly due to very low number of relapse incidence. Our outcomes were similar in terms of local control and survival when compared to published series, in which VBT was delivered with 7 Gy × 3 fractionation, but with longer overall treatments time. Therefore, the shortening of overall treatment time of exclusive VBT by adopting the schedule of 7 Gy each, every other day, in one week, up to 21 Gy was not detrimental in terms of survival outcomes. The critical issue was about the supposed increase of incidence of acute and, above all, late toxicities potentially related to the shortening of overall treatment time. In our study, after a median follow-up of 48 months (range, 15-119 months), no late G3-G4 toxicities were recorded. The incidence of acute and late G1-G2 toxicities, namely vaginal toxicities, were not dissimilar when compared with other reported series of endometrial cancer patients treated with exclusive VBT, as presented in Table 2. The recorded vaginal toxicity incidence in the present study may be related to several factors, especially the 3 cm of active length of treatment that was included in the CTV. This is consistent with studies by other authors, in which vaginal complications were associated with the length of vagina treated [12,30]. Moreover, systematic long-term use of vaginal topical therapy (2 times per week life-long or until insufficient tolerance/compliance) with hyaluronic acid could have a role in controlling the incidence of acute and late vaginal toxicities in our patients, as indicated by others [21]. In our patients, the use of vaginal dilator was not suggested since an adequate compliance with these devices could not be guaranteed in our patients due to their lack of specific education and training in this matter [31]. We were aware of several limitations resulting from a single canter retrospective analysis. Nevertheless, the data reported in this cohort showed excellent local control and low incidence of acute and late toxicities, comparing very favorably with other published reports. A prospective trial should be considered to validate our outcomes.