Purpose
Uveal melanoma is the most frequent primary malignant intra-ocular tumor in adults, and in majority of cases (82.5%) is located in the choroid. The risk of developing this tumor increases with age, Caucasian race, light-colored eyes, dysplastic nevus syndrome, oculodermal melanocytosis, and BAP1 cancer syndrome [1]. Currently, the most frequent treatment methods include radiotherapy and surgical interventions. The choice of treatment method depends on location and size of the tumor, presence of extra-ocular infiltration, and risk of complications and their influence on the maintenance of functional visual acuity. Prospective surgical intervention involves surgeries aiming at tumor resection (tumor endo- or exoresection) as well as more radical procedures, such as enucleation (eyeball removal). Radiotherapy is the most frequently used method of conservative treatment of uveal melanomas [2]. There are two main types of uveal melanoma radiotherapy: 1. Brachytherapy (BRT) that uses ocular applicators with radioisotopes, such as iodine-125 (125I), palladium-103 (103Pd), cesium-131 (131Cs), ruthenium-106 (106Ru), and strontium-90 (90Sr); and 2. Teleradiotherapy with external beam radiotherapy (EBRT) with photons (from megavolt linear accelerator), or EBRT with charged particles using protons or helium ions (from high energy cyclotrons) [3-7].
Brachytherapy and proton therapy show similar results of local control with 95-98% [3, 8-11]. However, long-term patient survival with uveal melanoma is approximately 50%, and considering the risk of generalization of the process, most frequently in the form of liver metastases (90%) [1].
Brachytherapy is a method that has been successfully used for more than 50 years. It consists of suturing an applicator with a radioisotope onto the surface of sclera above the base of tumor. The placement of an applicator close to the tumor base results in the supply of an appropriate dose of irradiation into cancer tissue with a simultaneous decrease of irradiation to surrounding healthy tissues. This method, however, has some limitations in the location of tumor in the optic disc, and large size of the tumor base or its thickness.
Presently, in our department of clinical ophthalmological oncology, ocular applicators contain 106Ru and 125I radioisotopes [12]. For the treatment of melanomas not thicker than 5 mm, radioactive ruthenium (106Ru) is used, while for tumors larger than 5 mm, iodine (125I) is applied [13]. In tumors with a thickness larger than 12 mm and base diameter exceeding 18 mm, enucleation is indicated [10, 14, 15]. However, in some cases, conservative treatment may be administered, i.e., brachytherapy, for tumors with a large base diameter of more than 18 mm. In general, this method consists of suturing an applicator onto the eyeball, covering some part of the tumor base. After reaching an appropriate dose, the applicator is shifted into the remaining part of tumor base. Exposition time is compliant with treatment plan calculated for the next 2 stages, which allows to cover an appropriate area with designated irradiation dose.
The objective of the current paper was to present the treatment method with the applicator displacement, which could be considered an alternative for enucleation in the treatment of uveal melanoma with a base larger than 18 mm.
Material and methods
Inclusion criteria for treatment with applicator displacement
Between 2012 and 2021, 13 patients who did not consent to enucleation were qualified for treatment with applicator displacement, and in some cases, this was connected with the presence of a tumor in the only eye with vision. The following four cases were excluded from the analysis: first patient due to two independent metastatic tumors in one eye; second due to diagnosis of intra-ocular lymphoma; third, in whom applicator displacement was a result of melanoma location around the optic disc; and fourth due to lack of follow-up (patient did not attend any follow-up visit). Finally, data from 9 patients were analyzed. In these cases, the criterion for the application of this type of treatment was a result of largest base diameter measurement, increased by approximately 2 mm margin around intra-ocular tumor, which was larger than the diameter of ocular applicators used: 20.5 mm for applicators with 125I seeds (POLATOM, Poland), and 20.2 mm for 106Ru applicators (Eckert & Ziegler BEBIG, Germany).
The correct displacement of the applicator during treatment allowed for complete coverage with isodose (80 Gy) of the entire tumor with a safe margin, assuming planned dose to be applied to the tumor apex was 80-120 Gy.
Retrospective analysis evaluated local treatment results of treating intra-ocular tumors with brachytherapy and displacement of the ophthalmic applicator. The paper discussed also physical parameters of treatment plans of large uveal melanomas with the displacement of the ophthalmic applicator in selected cases.
Study group
The retrospective analysis included 9 out of 13 patients with large intra-ocular tumors treated with brachytherapy.
In all 9 patients qualified for analysis, uveal melanoma was diagnosed on the basis of clinical status. Among the evaluated patients, there were 5 women (age range, 48-72 years; mean age, 61.6 years) and 4 men (age range, 45-83 years; mean age, 62.6 years). Follow-up time was ranging from 6 months to 6.6 years. The median whole follow-up was 2.9 years, and for patients with positive primary treatment results, it was 1.7 years. The median time to local relapse was 2.3 years. Clinical data is presented in Table 1. In Figures 1 and 2, the images show the stage of advancement of a patient who has been under control for the longest time, i.e., over 5.2 years.
Fig. 1
Ultrasound images of the melanoma before (A), two (B), and four (C) years after the treatment, showing a part of the tumor located in the posterior pole and in the area of the equator
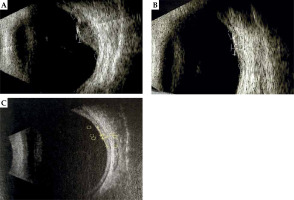
Fig. 2
Picture of the eye fundus before (A) and four years after treatment (B) of large uveal melanoma using applicator displacement
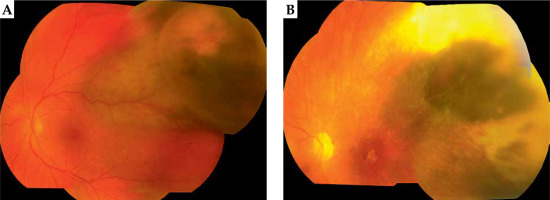
Table 1
Comparison of the study group
Treatment plan
Treatment plan was prepared with Plaque Simulator X system, version 5.3.9 (Eckert & Ziegler BEBIG, Germany) based on imaging data obtained from ultrasound (USG), UBM, or ocular fundus diagram. Ultrasound was used to measure meridian and longitudinal dimensions of the largest tumor base of intra-ocular tumor and its thickness. Ocular fundus diagrams allowed to evaluate the location of tumor and develop the position of applicator for suturing. During surgery after transillumination or fundoscopy, the largest tumor diameter measurements were verified, and the initial plan of the treatment could be modified according to intra-operative measurements before suturing the applicator.
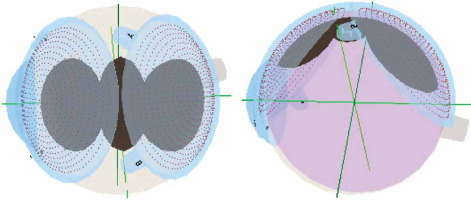
In order to select appropriate treatment parameters, i.e. the duration and applicator activity for a specific case, a simulation of dose distribution was made depending on tumor location within the eye. Tumor division into 3-4 independent smaller segments was simulated (Figure 3). The use of simulations for a segmented tumor allowed to determine doses in different places of the sclera and its apex (Figure 4). For each of the segments, a dose for the tumor top was assessed (total dose prescription point), which varied between 80 and 120 Gy, with doses per sclera in the range of 174.8 to 506.5 Gy. In addition, doses for peripheries of the treatment were verified (treatment margin) as well as doses in areas exposed to both applicators positions. In planning, the coverage of the entire tumor with its margin with the planned dose of 80-120 Gy for the tumor top was also established. While selecting treatment duration for specific applicators, decisions were guided by an uniform distribution of the dose within tumor volume. In differentiation of applicators at the disposal, there were significant differences in the treatment duration, which varied between 43 and 115.5 hours per one tumor fragment. Only one displacement was made during the treatment.
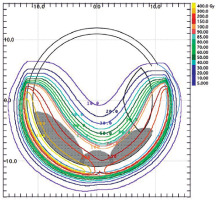
Fig. 4
Treatment plan: the scope of covering the tumor with isodoses after 106Ru applicator displacement
Round ophthalmic applicators were used with a diameter of about 20 mm. Suturing of the applicator containing radioactive isotopes (125I or 106Ru) was performed in an operating theatre, with local anesthesia and sedo-analgesia applied. In each of the investigated cases, the size of the tumor base and its location were visualized with transillumination as a shadow on the sclera. It is the practice at our center that transillumination is the primary tool to determine tumor basis prior to applying ocular applicator. If transillumination fails or we are unsure of tumor borders, we define them using fundoscopy.
During the first surgery after locating the base of tumor, the applicator was fixed to the sclera over one part of the base of lesion. Then, during the second treatment after re-localization of the base, the same applicator was moved to another non-irradiated part of the tumor base according to a previously prepared plan. The third surgery was the removal of the plaque. Accumulative treatment durations did not exceed 213 hours. Compilation of physical data on radiation exposure is shown in Table 2.
Table 2
Compilation of physical data on radiation exposure
A reduction in the thickness and/or size of the tumor base during follow-up was considered a positive outcome of local treatment.
Considering that any local recurrence or delay in effective treatment increases the risk of metastasis in uveal melanoma, we inform the patient of this by presenting various treatment options with their advantages, disadvantages, and consequences. However, ultimately, under the current law, it is the patient who consciously chooses the type of treatment.
Patient qualification and treatment course were compliant with the procedures of intra-ocular neoplasms with brachytherapy treatment that are mandatory in our center and follow quality control systems of our university hospital. These procedures are in accordance with the nuclear law in force in our country.
Results
In 5 patients (56%) treated with the ophthalmic applicator displacement method, a positive result, i.e., local control of the tumor was achieved. In the remaining 4 cases (44%), a local recurrence developed. Within the follow-up period (range, 6-37 months; mean, 17.5 months), 4 patients underwent enucleation: 3 cases due to tumor progression and 1 due to radiation complications, despite the positive results of local treatment. One patient with recurrence was qualified for a secondary 106Ru brachytherapy with transpupillary thermo-therapy. As a result of the second treatment, a positive local result of the therapy was obtained. In the 4 remaining patients with positive local results after the first treatment, no recurrence of uveal melanoma was observed within the follow-up period (range, 7-62 months; mean, 23.1 months). Results presented in Figures 1 and 2 show the positive effects of treatment of the patient, who has been under control for the longest time, i.e., over 6.6 years.
After one year of follow-up, in one patient, BCVA improved after treatment, and in the other 4 patients, it has deteriorated and was 0.1 or less.
In four patients, melanoma involved the ciliary body, three of whom had positive local treatment results (Table 1). Results of imaging examinations (USG) with changes in dimensions of the tumor during subsequent follow-up of the patients are presented in Table 3. Five patients achieved a reduction in tumor base size and thickness after the first treatment. In another four cases, the base diameter or thickness increased during the first year of follow-up. In three patients with recurrences, an increase in tumor thickness was observed, and in the remaining one, the base of tumor grew, which made it necessary to qualify all the 4 patients for a second treatment. Three patients with negative local treatment results underwent enucleations, and one patient received secondary brachytherapy with supplemental transpupillary thermo-therapy (TTT).
Table 3
Dimensional change in ultrasound examination of neoplastic lesions during last follow-up visit
Among the study group of patients, there were complications due to the use of brachytherapy, with their nature largely depending on the location of neoplastic lesion, as in classic cases. Type and number of radiation complications in the analyzed group are shown in Table 4.
Table 4
Complications after brachytherapy treatment with applicator displacement (results from the last follow-up visit)
Melanoma dissemination to the liver was found on imaging examinations in 2 patients. These patients did not present for further follow-up at our clinic.
Discussion
Brachytherapy tumor treatment with applicator displacement is an attempt of conservative therapy, which might bring beneficial effects, such as deactivation of tumor cells that spares the eye and even its useful function. The analysis of treatment plans showed appropriate coverage of the entire tumor area, while preserving the margin of the irradiated area. The doses applied to the tumor top and to the sclera reached an appropriate values. To summarize, the only involvement of the optic disc with tumor infiltration and tumor thickness above 12 mm excluded brachytherapy treatment with applicator displacement as an alternative for enucleation or proton beam radiotherapy (PBRT). In case of large uveal melanomas with the base exceeding 18 mm, it is always useful to consider three scenarios of a treatment plan, including brachytherapy with intra-operative applicator displacement.
PBRT is an effective treatment for diffuse uveal melanomas, but it is not universally available, and not all patients select it due to inconveniences associated with its implementation. Moreover, results of local treatment with PBRT are comparable with brachytherapy [3, 6, 8-15]. In contrast, for many patients, the decision of removing an eyeball is difficult, especially in cases where the neoplasm is located in the only eye with vision. However, it is easier to make such a decision if it is preceded by an unsuccessful attempt of conservative treatment. In our opinion, presenting one more method of treating uveal melanomas with a base larger than 18 mm and thickness not exceeding 12 mm in the form of brachytherapy with intra-operative applicator displacement, is a very good option. In our analysis of a group of patients treated with this method, 56% had positive local outcomes of the treated tumor. Similar positive results of local treatment have been obtained with brachytherapy in a rare variety of uveal melanoma that is ring melanoma, where removal of the eyeball is the standard of treatment [16]. In three cases of recurrence, an increase in tumor thickness was observed; in the remaining one, the base of the tumor increased. In one case with marginal recurrence, this was most likely due to juxtapapillary location. An increase in tumor thickness was found at the site of greatest thickness before treatment. It can be concluded that these tumors were insensitive to radiation, as doses to the tumor apex were within therapeutic range of 80-120 Gy. Cases of failure of local brachytherapy for uveal melanomas in the form of an increase in tumor thickness are a constant aspect of treatment with this method. As reported in the literature, the success rate of local control of uveal melanoma after brachytherapy ranges from 82% to 98% [8, 12, 13, 17].
A local recurrence of uveal melanoma means a higher probability of systemic dissemination and death [18, 19]. According to the Ophthalmic Oncology Task Force, there is a significant difference in 5-year and 10-year survival after treatment of uveal melanoma between groups of patients with a relapse and without relapse (p < 0.001), with 71% and 62% as opposed to 87% and 82%, respectively [13, 19].
An uveal melanoma spread to the liver was found on imaging examinations in 2 patients. These patients did not present for further follow-up at our clinic.
Our information on patients’ survival is based on an analysis of follow-up data at our center, or sometimes on information obtained from patients or their relatives (e.g., telephone, e-mail). If the patient does not attend a follow-up appointment, we lose the possibility of monitoring the patient’s state of health. In our country, due to legal aspects, we do not have the option to follow-up patients who do not present themselves for the designated medical follow-ups. However, the rules of follow-up in our region have changed over the years. The current follow-up schedule at our clinic is 3, 6, and 12 months after treatment, and then once a year at most.
In the current study, melanoma involved the ciliary body in four patients, three of whom had positive local treatment results.
Based on retrospective studies, mortality rates for uveal melanoma for comparable sized tumors treated by enucleation or other globe conserving methods, such as radiotherapy, appear to be similar [20]. Also for this reason, the use of first-line conservative treatment in the form of applicator displacement brachytherapy in uveal melanomas with a base larger than 18 mm seems justified.
The presented cases show that brachytherapy with applicator displacement may be an effective way of treatment. It is worth observing whether in this therapy, a wider tissue margin around the tumor is irradiated, as this area can comprise a flat tumor infiltration, which might not be visualized in initial measurements, and can only be revealed in transillumination assessment prior fixation of the applicator to the sclera. The applicator displacement in large tumors allows for a reduction of high local doses of irradiation applied onto the sclera, which is a desired situation.
We are aware that the number of the analyzed group was small in our study, which makes further research of this method necessary. Our aim was to present an alternative treatment method for diffuse uveal melanomas in patients, who for various reasons did not undergo enucleation or proton therapy.
An analysis of studies did not show similar attempts of treatment with the use of the applicator displacement for treating intra-ocular melanomas. Therefore, the described cases may be regarded as one of the first treatment attempts of using this method in particular situations.
Conclusions
Brachytherapy with ocular applicator displacement allows for the treatment of uveal melanoma with base measurements larger than 18 mm. The application of this method may be considered as an alternative for eye enucleation in patients with large diffuse tumors, such as a neoplasm of the only eye with vision, or when a patient does not consent to enucleation despite being informed of the possible negative health consequences of such a decision.