Purpose
Brachytherapy is an essential component for the treatment of gynecological malignancies due to its ability to deliver higher doses to the target volume while minimizing the dose to adjacent organs at risk [1]. The Surveillance, Epidemiology, and End Results (SEER) analysis reported a 13% detriment in cause-specific survival (CSS) and a 12% detriment in overall survival (OS) with the omission of brachytherapy [2]. The advancement in implantation techniques, image-based target delineation, and the ability of modern applicators to cover large residual diseases has led to improvement in local control and resultant disease-free survival when compared with historical series [3-6].
A vast majority of image-guided adaptive brachytherapy series report a high-risk clinical target volume (HR-CTV) of 30-40 cc [5, 6] at the time of brachytherapy. However, up to 15% of patients with stage IIB-IIIB disease can have a poor response to chemoradiation with large residual disease in the distal parametrium or extending up to the lateral pelvic wall [7]. Though there is an expectation of high local control of > 90% in patients who achieve HR-CTV D90 > 85 Gy, in these sub-cohorts of patients, HR-CTV D90 of 70-75 Gy may be expected with hybrid intracavitary-interstitial applicators that have parallel needles only. Clinical implementation of a modified Vienna applicator involving intracavitary and interstitial parallel and oblique needles that included 69 patients from two institutions reported the feasibility of achieving higher D90 to the HR-CTV, in patients with large residual disease (median HR-CTV 71 ±34 cc (1-SD). Using parallel and oblique needles along with intracavitary implant, a median D90 of 86 Gy (65-107 Gy) could be delivered to the HR-CTV. This group of patients had 76% local control and an increased risk of grade 3 or 4 adverse events (20%). Another study reported comparative dosimetry with case examples using parallel and oblique needles. It reported improved dosimetric outcomes in patients with large residual HR-CTV [8]. Further individualized brachytherapy may be feasible with 3D printed strategies [9-11]. In some patients, despite all these techniques, it may still not be possible to cover the HR-CTV with doses > 70-75 Gy. Various institutions experienced in advanced brachytherapy techniques therefore use treatment individualization, including free-hand interstitial needle techniques [12], which can be adapted based on residual disease. However, limited descriptive data are available in the published literature on how to perform these advanced implants and the resultant dosimetry that can be achieved. Another potential challenge in these patients is an elevated risk of moderate to severe complications, both as a result of significant residual disease and complex implants. Similar clinical challenges to perform a good implant may exist in patients with vaginal cancer or locally advanced inoperable endometrial cancer who have large residual disease at the time of brachytherapy.
In this case series, we describe, through real case examples, techniques, doses, and outcomes in patients where commercially available intracavitary interstitial applicators were not suitable and personalized brachytherapy was performed.
Material and methods
Around 200 patients with gynecological malignancies are treated with brachytherapy in our institution annually. This case series consists of six highly selected patients who underwent individualized brachytherapy from 2021 to 2023. A total of three patients were diagnosed with primary vaginal cancer, one with cervical cancer, one with vulvar carcinoma, and one with inoperable endometrial cancer. All patients underwent a clinical examination at diagnosis and the findings were documented using clinical diagrams [13]. Baseline magnetic resonance imaging (MRI) of the pelvis defined the locoregional disease extent. All patients received external beam radiotherapy to the pelvis (extended field radiotherapy if clinically indicated) to a dose of 45 Gy in 25 fractions at 180 cGy per fraction delivered 5 times a week. Patients eligible for concurrent chemotherapy received weekly cisplatin at 40 mg/m2 (5 cycles). At the end of external beam radiotherapy, a clinical examination was performed to assess treatment response and determine the residual disease, and the type of brachytherapy applicator. A preplanning MRI of the pelvis (T2-weighted images) was obtained for preplanning. The individual steps involved in performing brachytherapy with complex anatomy or large residual disease are described in the subsequent sections. All these patients were counselled regarding the inability of surgical excision, anticipated complexity of the implant, and treatment outcomes including expected adverse events. Intraoperative transabdominal ultrasonography was used to guide the insertion of the central tandem and to obtain measurements of the gross tumor volume (GTV). The length of the needles from the perineum was recorded at the time of implantation to ensure that no displacement occurred during treatment delivery. HR-CTV and organs at risk were delineated using the clinical-radiological findings. Patients underwent CT-based planning with or without MRI performed with the applicator in situ.
The planning limit for prescribed doses for D90 HR-CTV was 85 Gy for patients with cervical tumors [5]. For primary vaginal cancers or disease involving the upper or middle vagina, the planning goal was to maintain a CTV D90 dose of 75-80 Gy, whereas for lower vaginal disease, the planning aim was set at 70-75 Gy. The prescription limit for bladder, rectal and sigmoid D2cc, and rectovaginal point EQD23 were 90 Gy, 75 Gy, 75 Gy, and 65 Gy, respectively. The maximum permissible dose for the posterior fourchette was 60-65 Gy, and for the urethra (D0.1cc) was < 85 Gy [14-16]. Manual dose optimization was preferred over the graphical method of optimization [17]. Equivalent doses in 2 Gy per fraction (EQD2) were used to calculate the cumulative doses. On the second day of treatment, involving a single application with multiple fractions, repeat CT imaging was performed to confirm applicator positioning, changes in adjacent organs at risk, and the need for reoptimizing the treatment plan if necessary.
Results
Patient 1
Clinical presentation
A 44-year-old woman was diagnosed with stage II vaginal adenocarcinoma (gastric type). The tumor was 4 × 3 cm and involved the lower one-third of the vagina, rectovaginal septum, and left paracolpos. The disease extended to the introitus, with no mucosal infiltration into the rectal lumen. Surgery was not planned due to the expected morbidity associated with a permanent stoma. She received concurrent chemoradiotherapy to a dose of 45 Gy in 25 fractions along with weekly cisplatin at 40 mg/m2.
Challenges encountered at brachytherapy
Upon completion of external beam radiotherapy, vaginal examination revealed large residual disease in the lower one-third of the vagina and close to the perineal skin with involvement of the rectovaginal septum and left paracolpos. Curative doses in this case would be much higher than the tolerance of adjacent organs at risk. Surgical consultation was obtained after external radiation. However, the patient was not willing to undergo posterior exenteration.
Brachytherapy implantation, planning, and dosimetry
After counselling regarding treatment outcomes and anticipated severe morbidity, including skin and posterior fourchette necrosis, she underwent template-based (Martinez Universal Perineal Interstitial Template – MUPIT) brachytherapy. Nineteen parallel needles and 3 divergent needles were used to ensure adequate disease coverage. The needles were also implanted through the posterior plate in a concavo-convex manner around the rectum to ensure coverage of the posterior and rectovaginal disease extent while minimizing the dose to the rectum. The dose was also extended up to the perineal skin (caudal disease extent at brachytherapy) through additional placement of bolus adhesive material, and loading of needles was performed to cover the caudal tumor extent adequately (Figure 1). With this implant the patient received 32 Gy in 6 fractions. Daily re-planning and manual dose optimization were performed. Treatment was delivered at least 6 hours apart over 4 days. HR-CTV was 82.8 cc. HR-CTV D90 of 75.7 Gy was achieved with bladder, rectum, and sigmoid 2 cc doses of 61.4, 77.2, and 45.3Gy, respectively (Table 1). The maximum point dose at the posterior fourchette was 68.2 Gy. The doses to the posterior fourchette and rectum exceeded the permissible limit to cover the target volume.
Fig. 1
Case vignette: Patient 1. A, B) Prebrachytherapy MRI images showing the bulky lesion involving the lower one third of the vagina and rectovaginal septum. Disease extends up to the posterior fourchette. C) Examination under anesthesia revealed similar findings of thick 3 × 4 cm residual gastric adenocarcinoma lesion reaching up to the introitus and posteriorly, necessitating coverage of the dose right up to the introitus and skin. Note the use of adhesive dressing to help pull the isodoses to the surface. For visualization of disease extent at the time of implant, the impression of the disease is made on the surface. D) Template- based implant covering the disease involving the rectovaginal septum. In a routine gynecological patient, only the needle plane just posterior to the cylinder is used. In this patient, the caudal MUPIT plate was also used, and 3 additional planes of needles were implanted posteriorly not only to cover the disease but also to ensure concavo-convex dose distribution for rectal sparing. Nineteen parallel and three divergent needles were used. E) Clinical target volume (CTV) delineation; note the overlap between rectal wall outer contour and CTV as the disease involved the outer rectal wall. F-H) Final dose distribution achieved. Note the sparing of the rectum while ensuring adequate target volume coverage. This is also reflected in the two years clinical outcome of the patient, who has no rectal symptoms
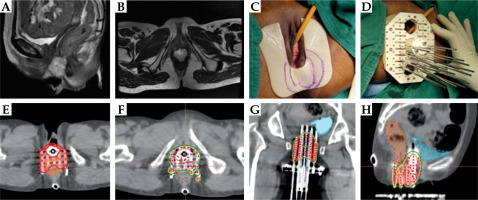
Table 1
The dosimetric details and outcomes of cases
Outcomes
The patient completed the planned implant in January 2023. After brachytherapy, she developed desquamation in the perineal region overlying the diseased surface, along with skin infection. At 3 months, she had a complete metabolic response on positron emission tomography with computed tomography (PET/CT). She had mild slough at the posterior fourchette for which hyperbaric oxygen was initiated for 30 sittings, and the soft tissue and skin showed recovery. The patient had no gastrointestinal, rectal, or urinary late adverse events. After 32 months, she remained locally controlled. However, she developed two lung metastases for which she was planned for stereotactic radiation in January 2025, followed by systemic chemotherapy. After completion of chemotherapy, she remains disease-free in August 2025.
Patient 2
Clinical presentation
A 39-year-old woman was diagnosed with cervical squamous carcinoma stage IVA (bladder and rectal involvement). The baseline clinical examination revealed a 4 × 4 cm exophytic lesion involving both cervical lips and fornices. The upper one-third of the anterior vaginal wall was involved. The left parametrium was involved up to the lateral pelvic wall, along with a uterosacral ligament deposit up to the lateral pelvic wall. Due to extensive disease at presentation that was not suitable for curative intent treatment, four cycles of paclitaxel and carboplatin were administered every three weeks as neoadjuvant chemotherapy. The response assessment, MRI, and cystoscopic examination did not reveal any bladder or rectal infiltration. Thereafter, based on the partial disease response, she was considered for curative intent external beam radiotherapy with concurrent chemotherapy using the intensity-modulated radiation therapy (IMRT) technique.
Challenges encountered at brachytherapy
After external beam radiation therapy (EBRT) (45 Gy in 25 fractions) with concurrent chemotherapy, vaginal examination revealed a persistent infiltrative disease in the left anterolateral vaginal wall and left parametrial infiltration of the uterosacral up to the lateral pelvic wall. At the time of brachytherapy, her uterus had sharp anteversion and persistent tumor deposits along the left piriformis muscle (in a direction that was different from the uterine and parametrial orientation). No standard applicator could potentially cover the disease completely.
Brachytherapy implantation, planning, and dosimetry
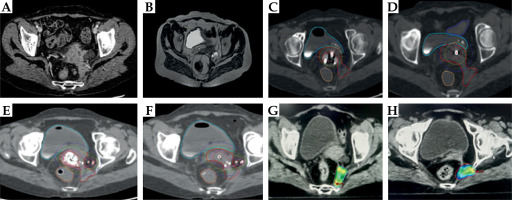
The first implant was performed using the tandem-ovoid applicator to understand the anatomical relationship of the disease with the applicator. However, due to unacceptable HR-CTV doses in the first application, the procedure was abandoned without treatment, and she was planned for hybrid brachytherapy. A Geneva applicator with a central tandem length of 60 mm, tandem angle of 15 degrees, 15 mm bilateral ovoids, and interstitial needles was used. Additionally, free-hand needles were implanted along the left posterolateral parametrium (n = 6). Brachytherapy was delivered to a dose of 2800 cGy in 4 fractions, applied twice daily, 6-8 hours apart. The lateral parametrial deposit was however not completely encompassed with interstitial brachytherapy. Therefore, she was further planned for an external beam radiotherapy boost (6 Gy/3 fractions). This was carefully performed, ensuring overlap of only a 50-70% isodose (Figure 2). With the combination of brachytherapy and external beam radiotherapy boost, higher target coverage with an acceptable OAR dose gradient could be achieved. For HR-CTV of 57 cc the cumulative D90 was 74 Gy with bladder, rectum, and sigmoid 2 cc doses of 90 Gy, 80.6 Gy, and 70.6 Gy, respectively (Table 1).
Fig. 2
Case vignette: Patient 2. A) Pretreatment CT scan showing disease involving left uterosacral ligament and deposit near left piriformis muscle. B) Post-EBRT MRI showing partial disease response with persistent left parametrial, uterosacral, and pyriformis muscle tumor deposit. C, D) First intracavitary application with Fletcher suit applicator showing poor HR-CTV coverage. E, F) Second brachytherapy application using a 15 mm Geneva applicator and free-hand interstitial needles along the left parametrium. Note that there is an improvement in coverage of lateral parametrial disease. However, there is suboptimal coverage of the left piriformis muscle deposit. G, H) IMRT boost to the left pyriformis tumor deposit
Patient 3
Clinical presentation
A 30-year-old woman was diagnosed with squamous cell carcinoma of the vagina, FIGO stage IVA (anterior rectal wall involvement). Baseline clinical findings revealed an infiltrative lesion involving the entire vagina from the 11 to 8 o’clock position with caudal extension up to the introitus. The lesion involved the peri-urethral region and posterior fourchette. The left parametrium was involved up to the lateral pelvic wall (LPW), while the right parametrium was free. Baseline images also revealed a low-lying position of the cervix (mid-vagina), suggesting uterine prolapse. She received external beam radiation to a dose of 45 Gy in 25 fractions over five weeks, along with concurrent chemotherapy.
Challenges encountered at brachytherapy
Examination under anesthesia revealed a hard and fixed residual lesion in the vagina at 1 cm from the introitus along the 1 o’clock to 8 o’clock position. The vagina was short, with a total length of 4 cm due to prolapse of the uterus and cervix. There was an ulcerative, excavating disease in the left upper vagina with necrosis that extended up to the bony wall.
Brachytherapy implantation, planning and dosimetry
A ring applicator with parallel and divergent needles was used (CT – 6 cm, angle – 15°). Due to the excavating ulcer in the lateral wall of the vagina, transvaginal access of the needles was avoided, and 6 trans perineal needles were inserted using a perineal template (Figure 3). Due to the complexity of the anatomy, the presence of an excavating ulcer, and the need for a highly individualized procedure, a single implant and multiple fractions were delivered. In this patient, the HR-CTV was 45 cc, and the HR-CTV D90 was 77 Gy. The bladder, rectum, and sigmoid 2 cc doses were 88.6 Gy, 80.6 Gy, and 52.5 Gy, respectively (Table 1).
Fig. 3
Case vignette: Patient 3. A, B) Baseline MRI showing extensive disease involving the posterior vagina, left parametrium, and infiltration of the anterior rectal wall with cervical prolapse. C) MRI at the time of brachytherapy showing partial response and thick anterior and lateral vaginal wall disease. D) Trans-perineal needles covering the lateral and posterior extent of the disease. E, F) 100% isodose covering the entire extent of vaginal and parametrial disease. Note the excavating ulcer at the 3 o’clock position, which led to a decision not to implant needles from the vaginal route but through the perineal route. G, H) Coronal and sagittal sections of the brachytherapy application show adequate coverage of HRCTV but also, due to the lower mid vaginal location of the entire implant, it was not feasible to correct the prolapse
Patient 4
Clinical presentation
This patient was diagnosed with stage IB vulvar squamous carcinoma in July 2017. She underwent a wide local excision with bilateral groin node dissection in August 2017. After a disease-free interval of 5 years, she presented with local recurrence in the periurethral region. Baseline examination revealed a proliferative growth along the anterior wall of the vagina submucosally in the periurethral region with involvement of the left labia minora, clitoris, and distal one-third of the urethra (cystourethroscopy). She completed external beam radiotherapy to a dose of 45 Gy in 25 fractions over 5 weeks along with concurrent chemotherapy.
Challenges encountered at brachytherapy
After external beam radiotherapy, she had a partial response with residual disease in the distal vagina, clitoris, and periurethral region. An individualized 3D printing template was used to cover the periurethral disease adequately. A 3D-printed template was created after obtaining a disease impression for brachytherapy preplanning.
Brachytherapy implantation, planning, and dosimetry
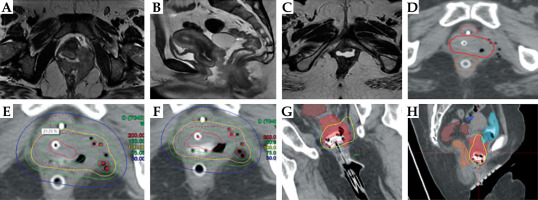
She underwent brachytherapy implant with this 3D-printed clitoral, periurethral, and vaginal template with a multichannel cylinder. Two horizontal interstitial needles were implanted free-hand in the clitoris and replaced with plastic catheters (seen as pink beads in Figure 4) and further supplemented with 3 horizontal needles on the template. Additional needles were also implanted around the Foley’s catheter to ensure urethral coverage. She received 5 fractions of 350 cGy each, treated twice a day, at least six hours apart. The total HR-CTV was 17.1 cc; however, due to the complex implant and heterogeneity of the target, the D90 was 65 Gy. The anorectum D2cc was 50.1 Gy, and the urethra received a maximum dose (D0.1cc) of 96 Gy (Table 1). After completion of the implant, applicator removal was performed under anesthesia due to the critical location of all the needles. Foley’s applicator was left in situ for an additional week as post-brachytherapy edema was expected.
Fig. 4
Case vignette: Patient 4. A) Baseline MRI showing the periurethral lesion and extension into the distal one third of the urethra. B) Post-EBRT MRI showing partial response at the primary lesion. C) Residual periurethral disease at the time of brachytherapy implant. D) Horizontal implantation of brachytherapy tubes to cover the disease over clitoris and left labia minora. E) Use of personalized 3D printed periurethral plastic template. Four interstitial plastic needles were placed in the periurethral region. F) Clinical target volume (CTV) with respect to the implant. G, H) Final dose distribution after manual dose optimization. The periurethral as well as the vaginal disease is covered adequately
Patient 5
Clinical presentation
A 41-year-old woman was diagnosed with primary vaginal cancer stage IVA (rectal involvement). At diagnosis, she had a large exophytic growth predominantly arising from the posterior vaginal wall. The cervix could not be visualized. The baseline MRI showed a large lobulated lesion arising from the lateral vaginal wall and involving both anterior and posterior walls, with loss of fat planes with the anorectum.
Challenges encountered at brachytherapy
After completion of concurrent chemotherapy and external radiation to a dose of 5000 cGy in 25 fractions, vaginal examination revealed a thick residual disease with the epicenter in the right vaginal wall extending from the 5 to 11 o’clock position. The disease was palpable 2 cm above the introitus and extended cranially for 4 cm without involving the cervix. The disease also extended to the right paracolpos. Silver markers were placed at the caudal end of the disease and the posterior fourchette for identification of mucosal disease extent. The large residual disease did not allow placement of even the smallest diameter applicator in the vagina. The cervical os could also not be localized.
Brachytherapy implantation, planning, and dosimetry
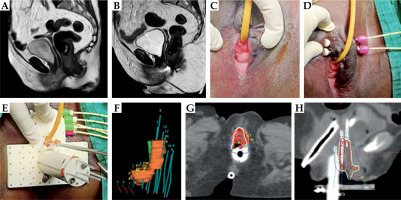
For the first brachytherapy application, the aim was to obtain some disease regression to allow placement of the brachytherapy applicator. Hence, we used only the right semi-ovoid of the 22 mm Venezia applicator along with a vaginal cap (due to the narrow vaginal space), along with three parallel interstitial needles on the right side. The 200% isodose was expanded alongside the residual tumor to achieve disease regression, and the patient was considered for BT again after a week. To achieve further tumor regression, she was planned for one more application using the narrowest multichannel MRI compatible vaginal cylinder with a central tandem. For the third application, we used a multichannel MRI compatible vaginal cylinder along with 6 free-hand needles in the right paracolpos (Figure 5). The HR-CTV was 65 cc. With the combination of implants, we could achieve HR-CTV D90 of 69 Gy, whereas bladder, rectal, and sigmoid doses were 81.3 Gy, 82.9 Gy, and 66.9 Gy, respectively (Table 1).
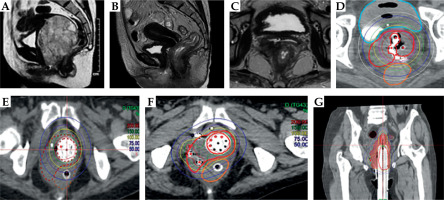
Fig. 5
Case vignette: Patient 5. A) Pre-EBRT MRI showing large lobulated mass arising lateral and posterior vaginal wall with loss of fat planes with rectum. B, C) Response assessment MRI showing good response in the primary disease. However, note the thick residual disease involving the right posterolateral vaginal wall with limited vaginal space. D) First brachytherapy implant with right semi-ovoid of 22 mm Venezia along with vaginal cap and three parallel needles. The 200% isodose was expanded to achieve adequate tumor regression to facilitate advanced application in subsequent fractions. E) Second brachytherapy application using multichannel cylinder applicator to achieve further tumor regression. F, G) Axial and coronal sections of third brachytherapy application using multichannel vaginal cylinder and six free-hand transperineal interstitial needles placed along the right paracolpos to increase the lateral CTV coverage (the red dots indicate active dwell positions)
Patient 6
Clinical presentation
A 53-year-old woman presented with a large uterocervical mass with a small mass also felt in the pouch of Douglas. Biopsy was suggestive of p53 mutant high-grade endometrial serous carcinoma. On imaging, in addition to the extensive primary lesion involving the uterosacral ligaments, there was a 2.6 × 2 cm mesorectal node. Surgery was not considered feasible, and a multidisciplinary decision was made to proceed with concurrent chemoradiation. After completion of chemoradiation, she was evaluated for surgical resection. However, she was not considered suitable for surgery as it would involve considerable morbidity.
Brachytherapy implantation, planning, and dosimetry
She was planned for a combined intracavitary-interstitial implant with ring applicator with parallel and divergent needles as the first implant. In the subsequent implant, additional free-hand needles were placed to target the mesorectal nodal deposit with brachytherapy as surgery was expected to entail significant morbidity. Furthermore, an external boost would expose the rectum to high doses (Figure 6). The CTV for primary disease was 30 cc (excluding the nodal target volume). The D90 for CTV was 82 Gy, whereas the node received a total of 53.5 Gy (1.7-1.9 Gy with each brachytherapy implant). The 2 cc doses of bladder, rectum, and sigmoid were 86 Gy, 76 Gy, and 74 Gy (Table 1).
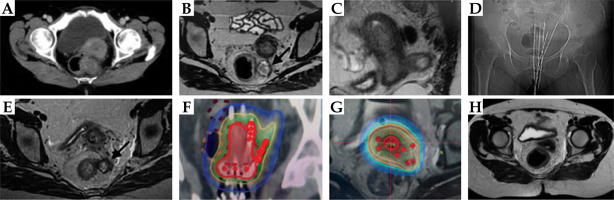
Fig. 6
Case vignette: Patient 6. A) Baseline CT scan showing utero-cervical disease and large mesorectal node. B, C) Prebrachytherapy MRI shows partial response at both primary and mesorectal node. D) Topography of the brachytherapy implant showing the needle placement. E) MRI with applicator in situ. F, G) Brachytherapy dose distribution showing good coverage of primary, parametrial disease, and needles close to medial part of the mesorectal node, depositing a sufficient dose for the nodal boost (1.7-1.9 Gy with each fraction). The rectum was carefully spared through treatment optimization. H) Response MRI at 6 months showing complete response. Patient continues to have no residual disease now at 50 months of follow-up
Outcomes
She completed the planned treatment in June 2021 and then proceeded to receive 4 cycles of systemic chemotherapy (paclitaxel-carboplatin). After completing systemic chemotherapy, she had a negative PET/CT, and surgery was deferred. She was last evaluated in April 2025 and continues to be free of any disease or treatment-related adverse events at 50 months of follow-up.
The dosimetric details and outcomes of each of these cases are summarized in Table 1.
Discussion
The published outcomes of image-guided brachytherapy in large, multi-institutional series report excellent results, with local control rates of upwards of 88% even in patients with stage IIIB-IVA disease [5]. Often in published series the target volumes and clinical outcomes are reported as medians, with little information about the clinical outcomes of patients who have large HR-CTV. Jamadagni et al. reported on a clinical series in which all patients received intracavitary interstitial with parallel and oblique needles [12]. The median HR-CTV volume in poor responders was 33 cc. Adaptive intracavitary with interstitial brachytherapy (IC/ISBT) resulted in 3-year local control (LC) and overall survival (OS) of 83% and 72%. The present case series with these case vignettes represents poor responders (HR-CTV median – 51.1 cc; range 17.1-82.18 cc) or those with challenging anatomy where any standard applicators could not cover the residual disease with an adequate dose. In each patient, the implant had to be individualized. As seen in the clinical outcomes of these patients, we achieved local control in only 3 out of 6 patients. Of the three patients who had disease progression, there was also a communicating fistula formation. In the remaining three patients who had disease control, one had late morbidity (posterior fourchette necrosis at the 6 o’clock position at introitus). This patient has only minor complaints and does not report a significant impact of these symptoms on her quality of life. Therefore, of the six patients, major morbidity was observed in 50% of patients. Patients who attained local control have had no local disease relapse for up to 2 years. While these results highlight the complexity of the clinical situation and the excellent possibilities of advanced brachytherapy, they also indicate that there is an unmet clinical need with the commercially available applicator design for this cohort of patients. Brachytherapy in these patients requires expertise, improvisation, and the integration of 3D-printed technology. In some patients with a very poor response, achieving a sufficient dose (HR-CTV D90 > 80-85 Gy) may still be a challenge and could be a potential contributor to disease progression and fistula formation.
Although these detailed case examples provide us with information on the techniques adopted for each of these implants, it is also important to consider whether these patients could have been alternatively treated with techniques such as spatially fractionated radiotherapy (SFRT), which may have yielded a superior therapeutic ratio. Pilot dosimetry studies investigating in silico dose distribution using straight and bending rods suggest the possibility of achieving doses similar to brachytherapy with desirable dose heterogeneity within the HR-CTV [18]. Also, in recent years, 3D printing technology has allowed better implantation of very advanced tumors [19]. Centers that have access to 3D printing technology could also have the possibility of improving dose coverage by using interstitial needles in a more controlled manner [20]. An MR suite for intraprocedural guiding and electromagnetic needle tracking could be of high value; however, these technologies need evolution and testing for gynecological hybrid brachytherapy [21]. Recently, the results of thermobrachytherapy have also been reported [22-24]. However, in this retrospective cohort, no additional benefit of hyperthermia was detected [25]. Furthermore, radiation sensitizers that could potentially be integrated with brachytherapy would also be of potential interest. Two recent developments in this direction have potential for further investigation: intratumoral injections of hydrogen peroxide or activated guided radiation by X-ray (AGuIX) nanoparticles [26-28].
While our experience demonstrates that interstitial brachytherapy may offer meaningful local control in selected complex gynecologic cancer cases, such approaches must be undertaken with great caution, as the therapeutic window is very narrow. A thorough assessment in a multidisciplinary tumor board setting is strongly advised before treatment. Informed consent should be detailed, clearly addressing potential benefits, risks, uncertainties, and limitations. These treatments are best undertaken at high-volume centers with access to diverse applicators and teams experienced in complex brachytherapy procedures and multidisciplinary management of complications. This ensures that the benefits of attempting organ- or function-preserving approaches are balanced against the responsibility to maintain the highest standards of patient safety and informed decision-making. We acknowledge that our analysis is limited by the small sample size, which precludes broad generalization; however, we believe that transparently reporting these rare and complex cases provides valuable clinical insights and may help guide decision-making in similarly challenging scenarios. Another limitation of our report is the relatively short follow-up in some patients, which restricts our ability to assess long-term outcomes and late toxicities.
Though further applicator developments in the field of brachytherapy will be actively pursued in future, our results provide information on the current unmet clinical needs of these poor responders and patients with challenging or narrow vaginal anatomy. As can be seen in the dosimetry of these patients, we achieved a total EQD2 of 65-82 Gy to the target volume. With the observed morbidity rates, it is unlikely that further dose escalation of tumor targets could be achieved without escalating organ-at-risk doses. Acceptability of morbidity in survivors with very advanced disease at brachytherapy needs active discussion and consent for patient acceptability of moderate to severe symptoms.
Conclusions
The present case narrative illustrates, through clinical examples, how individualized brachytherapy can be used to treat patients with challenging case scenarios who would otherwise be subjected to palliative treatments alone. The results highlight a clinical care gap that cannot yet be met with brachytherapy alone in all patients. Further developments in individualized brachytherapy with integration of novel agents and techniques may improve outcomes.



