Summary
Elevated plasma concentration of TMAO occured to be independent and firm predictor of major cardiac and cerebrovascular events after acute myocardial infarction in the course of the 3.5 years follow-up. We assume that the main cause might be the correlation between TMAO concentration and increased platelet reactivity.
Introduction
Cardiovascular disease (CVD), including acute myocardial infarction (AMI), is the leading cause of death worldwide [1]. In 2009, the global healthcare costs for CVD were estimated at €106 billion [2]. Despite the progress in the pharmacological and interventional treatment of AMI, recurrent ischaemic events such as cardiovascular death, recurrent AMI, or stroke occur in ~10% of patients within 1 year of the initial AMI [3]. At present, there is no tool to predict recurrent major adverse cardiac and cerebrovascular events (MACCE) after AMI. Accumulating data shows that gut microbiome plays an important role in the pathogenesis of CVD [4–6]. Among gut microbial metabolites, trimethylamine-N-oxide (TMAO) and indoxyl sulphate (IS) are the focus of extensive research in CVD [7, 8]. TMAO originates from the liver, which oxidizes trimethylamine (TMA), a TMAO precursor produced by conversion of carnitine, betaine, and choline by intestinal symbiotic bacteria [9]. IS, in turn, is a metabolite of dietary tryptophan that acts as a cardiotoxin and uremic toxin [10]. For example, TMAO was shown to have a dose-dependent association with platelet reactivity and cumulative incidence of thrombotic events in a cohort of over 4000 patients presenting for elective cardiac evaluations [11]. TMAO was shown to enhance platelet responsiveness to multiple agonists (adenosine diphosphate, thrombin, collagen) by enhancing the release of calcium from intracellular stores [11]. Elevated serum TMAO levels were also predictive of thrombus formation in atrial fibrillation patients [12]. Similarly, IS was shown to promote arterial thrombosis in a rat model [13] and induce platelet hyperactivity, thus contributing to chronic kidney disease (CKD)-associated thrombosis in mice [14]. Furthermore, IS exacerbated fibrosis and proliferation of cardiomyocytes [15].
Aim
We hypothesized that plasma concentrations of gut microbial metabolites differ between patients with AMI and healthy volunteers, and plasma concentrations of metabolites may be used as biomarkers to predict MACCE after AMI. The goal of this study was to (I) compare plasma concentrations of TMAO and IS in patients with AMI and healthy volunteers, as well as in patients with AMI who did and who did not experience MACCE during the median follow-up of 3.5 years, (II) to evaluate the predictive value of TMAO and IS for MACCE, and (III) to assess the correlation between TMAO and IS and platelet reactivity.
Material and methods
Study design
This was a prospective, observational study including patients participating in the AFFECT EV Metabolite Substudy. AFFECT EV was an investigator-initiated, prospective study conducted at the 1st Chair and Department of Cardiology, Medical University of Warsaw, Poland [16]. The study protocol, designed in compliance with the Declaration of Helsinki, was approved by the Ethics Committee of the Medical University of Warsaw (approval number KB/112/2016), registered in the Clinical Trials database (NCT02931045), and published previously [17]. All participants provided written informed consent.
Study participants
Study inclusion and exclusion criteria are listed in Table I. Patients were eligible for enrolment if they were (i) admitted to the hospital due to the first ST-segment elevation of AMI (STEMI) or non-STEMI (NSTEMI) with an onset of symptoms during the previous 24 h, and (ii) underwent PCI with stent implantation. STEMI was defined as persistent ST-segment elevation of at least 0.1 mV in at least 2 contiguous electrocardiography leads, or a new left bundle-branch block [18]. NSTEMI was diagnosed in patients with typical anginal chest pain accompanied by ST-segment changes (ST depression, T-wave changes, transient ST elevation) on electrocardiogram and an elevation of cardiac troponin concentration in the peripheral blood [19]. When the study was initiated, patients with STEMI were pre-treated with clopidogrel before hospital admission. Because antiplatelet therapy with P2Y12 inhibitors affects platelet reactivity, only patients who received clopidogrel prior to PCI were enrolled in the study to obtain a homogenous study group. Because gut metabolites are excreted by the urinary tract, patients with CKD with estimated glomerular filtration rate (eGFR) < 45 ml/min/1.73 m2, calculated using the Modification of Diet in Renal Disease (MDRD) formula, were excluded [20]. Because the intestinal metabolism is affected by the state of gastrointestinal tract and its microbiota, patients with acute or chronic gastrointestinal diseases, autoimmune disease, treated with antibiotics within the last 2 months or taking dietary supplements within the last 7 days, were excluded from the study [21].
Table I
Eligibility criteria for the study
Healthy volunteers were recruited among the hospital stuff and included people aged 18–99 years, without any medical history of chronic diseases, chronic pharmacotherapy, acute gastrointestinal disease within the last month, antibiotic therapy within the last 2 months, and dietary supplements within the last 7 days.
Trial schedule and blinding
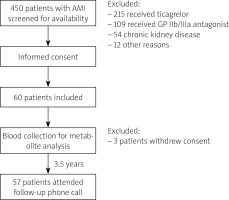
The trial schedule is presented in Figure 1. Blood was collected from patients by an independent operator (CE), who was otherwise not involved in sample analysis. Aggregometry was conducted by an independent operator (AG). During the trial, participants were identified by an individual number, and samples were coded with a sample number. Bacterial metabolite concentration analysis was performed by an independent operator, blinded to clinical data (MU). Statistical analysis was performed by independent operators (PS, KJ) [22].
Treatment
All patients received standard treatment after AMI according to the guidelines, including double antiplatelet therapy, β-blocker, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, aldosterone receptor antagonist, and protein pump inhibitor [18, 19].
Clinical data collection
Data collected at baseline include demographics (age, gender), body mass index, initial diagnosis, and cardiovascular risk factors, including arterial hypertension, diabetes, hyperlipidaemia, and smoking. In addition, routine laboratory parameters were recorded. At discharge, pharmacotherapy was recorded. Data regarding MACCE (recurrent AMI, stroke, cardiovascular death, all-cause death) were collected during a follow-up phone-call at a median of 3.5 years [22].
Blood collection and handling
Peripheral venous blood samples were collected from fasting patients at a single time-point (within 24 h after AMI). Briefly, blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes for metabolites analysis and into hirudin tubes for platelet reactivity analysis. The first 2 ml were disposed of to avoid pre-activation of platelets. Within a maximum of 15 min from blood collection, EDTA samples were centrifuged for 15 min at 2500 g. Plasma was frozen at –80°C until analysed [22].
Evaluation of gut bacterial metabolite concentration
The concentrations of TMAO and IS were measured using a Waters Acquity Ultra Performance Liquid Chromatograph coupled with a Waters TQ-S Triple-Quadrupole Mass Spectrometer. The mass spectrometer operated in the multiple-reaction monitoring (MRM)-positive electrospray ionization (ESI) mode, as we have previously described [23].
Platelet reactivity
Platelet reactivity was assessed by multiple electrode aggregometry using the adenosine diphosphate test (ADP, 6.5 μmol/l), and the thrombin receptor-activating peptide-6 (SFLLRN) test (TRAP, 32 μmol/l) was used as a positive control. Unstimulated whole blood was used as a negative control.
Endpoints
The primary endpoint of the study was the difference in plasma TMAO and IS concentrations between patients with AMI and healthy volunteers. The secondary endpoint was the prognostic value of TMAO and IS for the occurrence of MACCE during the median 3.5-year follow-up time. The exploratory endpoint was the correlation between TMAO and IS and platelet reactivity [22].
Statistical analysis
Statistical analysis was done using IBM SPSS Statistics, version 24.0 (IBM). Qualitative variables were presented as numbers and percentages and compared with the use of Fischer’s exact test. A Shapiro-Wilk test was used to assess the normal distribution of continuous variables. Continuous variables were presented as median with interquartile range or as mean and SD and compared using the Mann-Whitney U test or an unpaired t-test. The diagnostic ability of gut microbial metabolites to discriminate between patients with and without MACCE and the cut-offs were calculated using a receiver operating characteristic (ROC) curve. A logistic regression model incorporating the gut microbial metabolites with significant sensitivity and specificity (area under the ROC curve – AUC) and clinical characteristics was used to determine the best model for MACCE prediction. Mortality and other adverse events were reported depictively. A p-value below 0.05 was considered significant [22].
Results
Between January 2017 and July 2018, 60 patients were enrolled. Due to withdrawal of permission to participate in the study by 3 patients, 57 patients attended the final analysis and completed follow-up. Control group consisted of 27 age- and gender-matched healthy persons. MACCE was developed by 5 (8.8%) patients during the follow-up: 4 deaths (2 patients from unknown cause, 2 from cardiovascular cause) and a relapse of AMI.
Metabolite concentrations in patients with AMI and in healthy controls
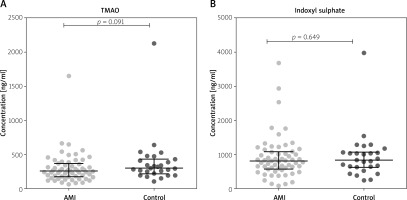
The concentrations of IS and TMAO concentrations were similar in patients with AMI and in the healthy controls (Figure 2).
Metabolites as predictors of MACCE after AMI
Table II shows the characteristics of patients who developed major adverse cardiovascular events and persons who did not. Patients with MACCE were older (p = 0.031), had increased creatinine levels (p = 0.001), and elevated levels of peak troponin I (p = 0.048) at baseline, in comparison to patients who did not experience MACCE. Nevertheless, the remaining cardiovascular risk factors and laboratory data were similar within the groups. Pharmacotherapy did not differ much between the patients. All of them received dual antiplatelet therapy – atorvastatin (except one person). The majority received a β-blocker, ACEI, and proton pump inhibitor (PPI).
Table II
Comparison of baseline characteristics between patients who experienced MACE and those who did not during the median follow-up of 3.5 years
[i] ACE – angiotensin-converting enzyme, ARB – angiotensin-receptor blockers, BMI – body mass index, weight in kilograms divided by square of the height in metres, CrCl – creatinine clearance, calculated according to the Cockcroft-Gault equation, CRP – C-reactive protein, Hb – haemoglobin, IQR – interquartile range, LDL-C – low-density lipoprotein-cholesterol, LVEF – left ventricle ejection fraction, NSTEMI – non-ST-segment elevation myocardial infarction, NT-proBNP – N-terminal pro-B-type natriuretic peptide, Plt – platelets, SD – standard deviation, STEMI – ST-segment elevation myocardial infarction.
The concentrations of intestinal microbial metabolites in the plasma of patients with and without MACCE after AMI during the 3.5-year follow-up are shown in Figure 3. TMAO (Figure 3 A) and IS (Figure 3 C) levels were elevated in patients who experienced MACCE, in contrast to those who did not develop any major adverse cardiovascular events (p ≤ 0.012 for all), and distinguished between the groups (area under ROC curve (AUC) ≥ 0.85, p ≤ 0.011 for all) in univariate analysis (Figures 3 B, D).
Figure 3
Gut microbial metabolites in plasma of patients with acute myocardial infarction predict major adverse cardiovascular events (MACE) during the median follow-up of 3.5 years. A, B – Trimethylamine-N-oxide (TMAO). C, D – Indoxyl sulphate
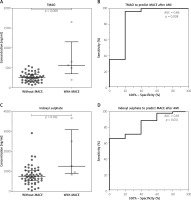
Table III indicates the statistical estimates of MACCE hazard via TMAO and IS, which also include the cut-off values, which were set based on the ROC curves. We include TMAO and IS in a logistic regression model alongside with patients’ gender and other clinical data (age, creatinine, troponin), to examine if these metabolites are independent MACCE predictors. In Supplementary Materials we have included a table with multivariable analysis, where MACE was used as a dependent variable, and predictors that were significant in univariable analysis were used as independent variables. The analysis indicates that the plasma concentration of TMAO at baseline was the only independent predictor of MACCE during the observation period (OR = 35.041, 95% CI: 1.269–967.307, p = 0.036), whereas the baseline concentrations of IS lost statistical significance to predict MACCE in multivariate analysis (OR = 9.260, 95% CI: 0.287–298.409, p = 0.209). This model, however, should be interpreted cautiously due to the small sample size (Table IV).
Correlation between platelet reactivity and SDMA
Figure 4 shows the correlation between plasma metabolites concentrations and platelets reactivity in response to ADP and TRAP in patients after AMI. Although there was no significant correlation between plasma TMAO and IS concentrations and platelet reactivity (Figures 4 A, B), patients with concentrations of TMAO and IS above the cut-off value predictive of MACCE (“high TMAO”, “high IS”) had higher platelet reactivity compared to patients with metabolite concentration below the cut-off value (Figures 4 C–F). However, the statistical significance was reached only for IS in the ADP test (p = 0.042; Figure 4 D).
Figure 4
Correlations between platelet reactivity in response to ADP and TRAP and plasma gut microbial metabolite concentrations (A, B) and comparison of platelet reactivity in patients with the concentrations of TMAO and IS above the cut-off value predictive of MACE (“high TMAO”, “high IS”), compared to those with metabolite concentration below the cut-off value (C–F)

Discussion
The main finding of our study is that the plasma concentration of TMAO was a firm and independent predictor of major adverse cardiovascular events after AMI in the course of the 3.5-year observation period, with 80% sensitivity and 96% specificity. Moreover, other studies indicate that elevated plasma levels of TMAO were associated with a higher risk of MACCE (death, myocardial infarction, or stroke) independently of conventional risk factors, for example chronic kidney disease, obesity, or diabetes mellitus, in stable patients with coronary artery disease managed with optimal medical treatment [24, 25] or undergoing elective coronary angiography [26], in patients presenting to the emergency department with chest pain [27], in patients after AMI [28], with chronic heart failure [29], and those after out-of-hospital cardiac arrest [30]. The association of plasma TMAO and adverse outcomes in cardiovascular patients was also confirmed in a recent meta-analysis [31]. Nonetheless, there are also studies that have shown no association between the plasma concentration of TMAO and adverse outcomes in cardiovascular patients, indicating that TMAO results tend to be confounded by impaired kidney function and poor metabolic control [32, 33]. In addition, it has been suggested that the TMAO precursor trimethylamine, but not TMAO itself, is involved in cardiovascular pathology by exerting negative effects on cardiomyocytes, probably due to disturbing their protein structure [7]. Hence, our study adds to the discussion in the literature on this topic.
In our study, the plasma concentration of IS predicted MACCE after AMI in univariate analysis, but it was not an independent predictor in multivariate analysis. In contrast to our results, other authors found that IS predicted adverse cardiovascular outcomes in patients after AMI and those with CKD undergoing dialysis [34, 35]. Again, further studies are required to determine the real prognostic value of IS for cardiovascular outcomes and potentially implement both TMAO and IS in daily clinical routine.
The association between plasma TMAO and IS concentrations and adverse cardiovascular events is multifactorial. First, chronic dietary L-carnitine supplementation in mice enhanced synthesis of TMA and TMAO by gut microbiota and increased atherosclerosis, thus contributing to the well-established link between high levels of red meat consumption and CVD risk [25]. There is evidence that an increased concentration of TMAO activates platelets and triggers clot formation, resulting in a higher risk of atherothrombotic events and cardiovascular death [11, 36]. In our study, patients with concentrations of TMAO above the cut-off value predictive of MACCE had higher platelet reactivity, compared to patients with metabolite concentrations below the cut-off value, although the result was not statistically significant. Nevertheless, based on previous evidence from the literature [35], an association between TMAO concentration and platelet reactivity might be one of the mechanisms underlying adverse outcomes.
The uremic toxin IS, in turn, was shown to activate inflammation and coagulation signalling pathways in the rat aorta in the short-term and induce calcification in the aorta and peripheral arteries in the long-term [37]. Furthermore, IS induced the production of reactive oxygen species and the expression of osteoblast-specific proteins in human aortic smooth muscle cells [38] and stimulated the proliferation of rat vascular smooth muscle cells [39]. These observations, derived from cell cultures and animal models, might at least partly explain the association between extensive calcification and faster progression of atherosclerosis in CKD patients. In our cohort of post-AMI patients without CKD (eGFR < 45 ml/min/1.73 m2) we could not confirm the independent predictive value of IS, suggesting that IS might be specifically used to predict adverse prognostic effects in CKD patients [40] but not in I CVD patients. Nevertheless, the main limitations of our study are the small sample size and wide confidence interval of TMAO predictive value in multivariate analysis. Therefore, we can only hypothesize rather than ultimately prove that an elevated concentration of TMAO in the plasma of AMI patients is related to MACCE. Accordingly, the data should not only be considered with caution but also confirmed in a larger study group.
The main limitation of our study is the small sample size and MACCE, which affects the statistical results and should be furtherly validated. We also did not assess the occurrence of MACE in the control group. Also, the impact of diet and PPI on gut microbiome and metabolites was not considered.
Conclusions
In our study elevated plasma concentration of TMAO was shown to be an independent and firm predictor of major adverse cardiovascular events after AMI in the course of the 3.5-year follow-up. We assume that the main cause might be the correlation between TMAO concentration and increased platelet reactivity. These results, however, may be affected by the small sample size and MACCE and are only hypothesis-generating.