Introduction
Severe pneumonia is a kind of pulmonary infectious disease in clinical treatment, with children presenting with coughing, confusion, and general malaise [1]. Due to the imperfection of lung development and poor physical resistance in children, patients are likely to have complications such as respiratory failure, resulting in unsatisfactory prognosis and increased mortality [2]. Respiratory failure refers to a severe lack of lung function, leading to a decrease in oxygen levels and an increase in carbon dioxide levels in the blood, which may cause damage to organs such as the heart and brain in children [3]. Medical experts indicate that failure to timely treat children with severe pneumonia can not only cause severe respiratory damage but may also affect growth and development, and subsequently may even be life-threatening [4, 5]. Therefore, it is crucial to explore effective biomarkers for early identification, timely diagnosis, and prognostic outcome assessment of children with severe pneumonia and respiratory failure.
MicroRNAs (miRNAs) are a class of small non-coding RNA molecules about 22 nucleotides in length, which are involved in the regulation of gene expression by binding to complementary sequences of target mRNA molecules [6, 7]. The role of miRNAs has received increasing attention in the study of diseases, and they are involved in the pathogenesis and progression of a variety of diseases, including tumors, respiratory diseases, cardiovascular diseases, and inflammation [8, 9]. For example, miR-146a was confirmed to mediate the antiviral response of recipient cells and participate in the molecular mechanism of viral infection, which may be a therapeutic factor for viral diseases [10]. miR-205-5p level was determined to be enhanced in COVID-19 patients, which may be associated with poor patient prognosis and immune response [11]. Moreover, miRNAs have been reported in mycoplasma pneumonia, respiratory infections, and immunodeficiency diseases [12-14]. miR-192-5p is located on chromosome 11q13.1 and is a member of the miR-192 family [15]. Evidence suggested that miR-192-5p was underexpressed in diabetic nephropathy and lung cancer, which affected the deterioration of the disease [16, 17]. In addition, miR-192-5p was also thought to be dysregulated in respiratory and digestive diseases and to play a role in the diagnosis and prognosis of different diseases [18].
Based on the fact that miR-192-5p has not yet been comprehensively studied in severe pneumonia, we aimed to evaluate the potential of miR-192-5p as a diagnostic marker for children with severe pneumonia and respiratory failure and its predictability for prognostic outcomes.
Material and methods
General information
A total of 62 children with severe pneumonia (age from 4 to 11 years) and 40 children with severe pneumonia with respiratory failure (age from 4 to 11 years) admitted to the Chinese and Western Medicine Hospital of Panzhihua from January 2023 to February 2024 were selected. In addition, a control group of 62 healthy children (age from 4 to 10 years) were also included. Inclusion requirements for children with severe pneumonia: 1) The children met the Pediatric Infectious Diseases Society/Infectious Diseases Society of America (PIDS/IDSA) guidelines for the diagnosis of severe pneumonia; 2) The children required mechanical ventilation; 3) The respiratory rate of children aged 1-5 years exceeded 40/min and that of children over 5 years old exceeded 20/min; 4) The children were dyspneic and unconscious. The requirements for inclusion of children with severe pneumonia and respiratory failure were: 1) The children met the diagnostic criteria for pediatric severe pneumonia combined with respiratory failure established by the WHO; 2) The respiratory rate was > 60 breaths/min with a heart rhythm of > 180 beats/min; 3) The children were pale and agitated. Healthy children were individuals with no abnormalities in all aspects after physical examination. Exclusion criteria: 1) Children were not suitable for early continuous positive airway pressure ventilation (CPAP) treatment; 2) Children with major cardiac, cerebral, or vascular diseases; 3) Children with congenital diseases or family genetic diseases; 4) Children whose families refused to participate or whose follow-up information was incomplete.
The program was approved by the Ethics Committee of Chinese and Western Medicine Hospital of Panzhihua and performed in line with the principles of the Declaration of Helsinki. All children’s legal guardians were informed about this study and signed informed consent.
Extraction of blood samples and detection of biochemical indicators
All the children involved needed to have their venous blood collected under fasting conditions, and promptly centrifuged (3000 rpm, 15 min) and supernatant extracted. The extracted supernatants were stored in a refrigerator at –80°C for subsequent experiments.
Biochemical parameters were analyzed by a fully automated biochemical analyzer (Beckman 5811, Germany).
RT-qPCR assay
Total RNA for miR-192-5p was extracted from serum samples by Trizol reagent (Invitrogen, CA, USA). cDNA was then obtained using the TaqMan microRNA Reverse Transcription Kit (Invitrogen, CA, USA). The RT-qPCR reaction system was configured according to the instructions of the TaqMan miR Reverse Transcription Kit (Applied Biosystems, USA) and quantified on an Applied Biosystems 7900 RealTime PCR system. The level of miR-192-5p was normalized by U6 and calculated by the 2-ΔΔCt method.
Prognostic evaluation criteria
The treatment effect and prognosis of children with severe pneumonia and respiratory failure were evaluated for 28 days after systemic treatment. The disappearance of lung rales, cyanosis, and dyspnea, and recovery of heart rate were regarded as a good prognosis, while the opposite was regarded as a poor prognosis, or the occurrence of complications such as dry mouth, abdominal distension, obstruction of sputum expectoration, and pressure congestion of the face were regarded as poor prognosis.
Statistical analyses
Data were processed and analyzed using SPSS 20.0 and GraphPad Prism 7.0 software. Measurements were expressed by mean ± standard deviation and analyzed by one-way ANOVA. Count data were expressed as n and subjected to chi-square analysis. The ability of miR-192-5p to differentiate between healthy and children with severe pneumonia was assessed by the ROC curve. Meanwhile, the predictive potential for the development of respiratory failure in severe pneumonia was also evaluated by the ROC curve. The Kaplan-Meier curve was used to describe the poor prognostic outcomes of children with severe pneumonia and respiratory failure. Furthermore, the risk factors of children with severe pneumonia and respiratory failure were analyzed by binary logistic analysis, and the poor prognostic factors of patients were determined by multivariate Cox analysis. P < 0.05 was considered statistically significant.
Results
Comparison of clinical indicators of included children
Comparing the general data, there were no statistically significant differences in age, gender, and body mass index (BMI) among the three groups (p > 0.05). The etiology for children with severe pneumonia included 8 cases of test negative, 4 cases of bacterial infection, 6 cases of viral infection, 26 cases of atypical pathogen, and 18 cases of mixed infection. For children with severe pneumonia and respiratory failure, there were 3 cases of bacterial infection, 5 cases of viral infection, 20 cases of atypical pathogen infection, and 12 cases of mixed infection. The etiology for both groups showed no significant difference (p = 0.220, Table 1). However, C-reactive protein (CRP), white blood cells (WBC), lymphocytes (LYM), neutrophils (NEU), and procalcitonin (PCT) were higher in the respiratory failure group than in the healthy and severe pneumonia groups (p < 0.001, Table 1).
Table 1
Inclusion of clinical indicators for children
Expression of miR-192-5p and its diagnostic properties
RT-qPCR assays revealed that miR-192-5p decreased in the severe pneumonia and respiratory failure groups compared with the healthy group, and the miR-192-5p level in the respiratory failure group was lower than that in the severe pneumonia group (p < 0.05, Fig. 1A). Moreover, miR-192-5p had high sensitivity and specificity (83.87% and 93.55%) for the prediction of severe pneumonia (AUC = 0.939, Fig. 1B). Meanwhile, the AUC was 0.907, with a sensitivity of 95% and specificity of 79.03% in identifying children with severe pneumonia and children with severe pneumonia and respiratory failure (Fig. 1C). Binary logistic analysis clarified that miR-192-5p is one of the risk factors for respiratory failure in children with severe pneumonia (p = 0.001, Table 2).
Fig. 1
Expression and diagnostic analysis of miR-192-5p. A) miR-192-5p expression in the serum of children with severe pneumonia was lower than that of healthy children and higher than that of children with severe pneumonia and respiratory failure (***p < 0.001 vs. healthy; ###p < 0.001 vs. severe pneumonia). B, C) ROC analysis indicated the predictive ability with miR-192-5p in severe pneumonia (AUC = 0.939) and respiratory failure (AUC = 0.907)
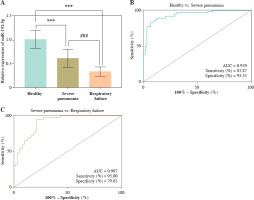
Table 2
Binary logistic analysis of children with severe pneumonia and respiratory failure
Association of miR-192-5p with prognosis of children with severe pneumonia and respiratory failure
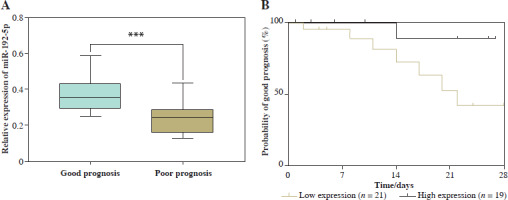
According to the prognosis of children with severe pneumonia and respiratory failure, 40 cases were divided into a good prognosis group and a poor prognosis group. miR-192-5p expression was lower in the poor prognosis group compared to the good prognosis group, determined using the RT-qPCR method (p < 0.05, Fig. 2A). In addition, the children with severe pneumonia and respiratory failure were categorized into a low expression group (n = 21) and high expression group (n = 19) based on the mean expression value of miR-192-5p. Kaplan-Meier curves revealed that the probability of a good prognosis for children with respiratory failure was decreased when miR-192-5p was lowly expressed (Fig. 2B). Additionally, miR-192-5p was identified as an independent prognostic factor for children with severe pneumonia and respiratory failure by multivariate Cox analysis (p = 0.025, Table 3).
Table 3
Multivariate Cox analysis of poor prognosis in children with severe pneumonia and respiratory failure
Fig. 2
Relationship between miR-192-5p and prognosis in children with severe pneumonia and respiratory failure. A) miR-192-5p level was downregulated in the poor prognosis group of children with severe pneumonia and respiratory failure compared to the good prognosis group (***p < 0.001 vs. good prognosis). B) Low expression of miR-192-5p indicated poor prognosis in children with severe pneumonia and respiratory failure
Discussion
The incidence of pneumonia has increased dramatically in recent years due to environmental pollution and the spread of the COVID-19 virus. Moreover, severe pneumonia is often induced in children because of the incomplete development of the lung tissue and the narrowness of the tracheal lumen, and the invasion of pathogenic bacteria or viruses [19]. As a serious lung infection, severe pneumonia affects the number of alveoli in children, causing ventilation and exchange dysfunction, as well as a systemic inflammatory response, which in turn leads to hypoxemia and dyspnea. In severe cases, children may develop symptoms related to respiratory failure [20]. For children with severe pneumonia and respiratory failure, early recognition, rapid treatment, and careful care are the keys to improving the cure rate and quality of survival.
The occurrence of severe pneumonia and respiratory failure causes damage to the immune system and inflammatory damage to the organism [21]. The biochemical parameters CRP, WBC, LYM, NEU, and PCT were higher in children with severe pneumonia than healthy children in this study, and the upregulation was more pronounced in children with severe pneumonia and respiratory failure. Furthermore, decreased miR-192-5p was found in serum samples of children with severe pneumonia and respiratory failure. An et al. noted that miR-192-5p was reduced in patients with gouty arthritis, and it improved the inflammatory response of patients by mediating the pathogenic process [22]. Fu et al. also noted a low level of miR-192-5p in diabetic retinopathy, which may serve as a developable marker for patient therapy [23]. These observations are similar to our findings. Meanwhile, ROC analysis reflected that miR-192-5p not only discriminated between healthy children and children with severe pneumonia but also better differentiated between children with severe pneumonia and respiratory failure children. miR-192-5p has shown early predictive ability in atherosclerosis, acute pancreatitis, and cervical adenocarcinoma [24-26]. Binary logistic analysis also illustrated that miR-192-5p was a risk factor for severe pneumonia in children with respiratory failure. These results imply that the downregulation of miR-192-5p expression may be accompanied by the aggravation of severe pneumonia, and miR-192-5p has the potential to diagnose severe pneumonia with respiratory failure.
Prognostic outcomes of children with severe pneumonia and respiratory failure were analyzed. Children whose lung rales and dyspnea disappeared and heart rate returned to normal after treatment were classified as the good prognosis group, and vice versa for the poor prognosis group [27]. miR-192-5p expression was lower in the poor prognosis group than in the good prognosis group. Kaplan-Meier analysis indicated that children with severe pneumonia and respiratory failure with low expression of miR-192-5p had a poor prognosis. Similarly, miR-192-5p may serve as a prognostic marker in pancreatic ductal adenocarcinoma and lung adenocarcinoma [28,29]. Additionally, multivariate Cox regression analysis revealed that miR-192-5p was an independent prognostic factor for children with severe pneumonia and respiratory failure. The above evidence suggests that low expression of miR-192-5p in children with severe pneumonia and respiratory failure is associated with a poor prognosis and unfavorable survival outcomes, potentially serving as a prognostic predictor.
Unfortunately, the present study still has some limitations. Firstly, this was a single-center, small-sample study with a limited sample size of patients, which may introduce bias into the results. Secondly, the study did not involve a discussion of molecular mechanisms, leaving the pathological mechanisms of respiratory failure not yet fully elucidated. Finally, cell or animal experiments were not represented in this study. Thus, the detailed mechanism of miR-192-5p in severe pneumonia should be explored by carrying out in vitro or in vivo studies. In future studies, we will further confirm the potential value of miR-192-5p in respiratory failure through molecular mechanisms and cellular experiments, which will provide a better reference for reducing the complications and mortality of children.
In summary, miR-192-5p level was reduced in children with severe pneumonia and respiratory failure, and miR-192-5p expression was downregulated in the poor prognosis group. Low levels of miR-192-5p have a high diagnostic value in children with severe pneumonia and respiratory failure and suggest a poor prognostic outcome for patients. Therefore, miR-192-5p may serve as a potential biomarker for the diagnosis and prognosis of respiratory failure in children.


