Introduction
Inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn’s disease (CD), is a non-specific, chronic, recurrent, and progressive inflammatory disease affecting the digestive system [1]. The underlying mechanisms and etiology of IBD remain incompletely understood; however, it is recognized that factors such as environmental influences, genetic predisposition, immune dysregulation, compromised intestinal mucosal barriers, and alterations in gut microbiota significantly contribute to its pathogenesis [2, 3]. Currently, it is considered that abnormal immune responses and hyperactivity play vital roles in IBD’s development, wherein environmental triggers act upon genetically predisposed individuals, ultimately contributing to the disease [4]. Predominantly affecting young and middle-aged populations, IBD manifests through persistent or recurrent diarrhea, abdominal pain, bloody and mucous stools, alongside various systemic symptoms. There is no clear standard for the diagnosis of IBD. The diagnosis is made on the basis of excluding other types of colitis by comprehensive analysis of clinical manifestations, laboratory examination results, imaging, endoscopy and histopathology [5]. Although clinical manifestations, imaging findings, and histopathological characteristics may differ between UC and CD, some patients exhibit features of both conditions, complicating clinical differentiation and necessitating long-term follow-up for accurate assessment. Consequently, there is a growing focus on developing simple and effective identification methods for IBD.
Currently, several serum biomarkers are integral to the routine laboratory diagnosis of IBD. However, due to their low specificity, these markers are primarily utilized for initial diagnosis and assessment. C-reactive protein (CRP), for example, is not only detected in the serum of patients with IBD but is also elevated in other autoimmune diseases, infections, and malignant tumors [6]. Long non-coding RNAs (lncRNAs), which are functional RNA molecules longer than 200 nucleotides, have been implicated in the pathogenesis of autoimmune diseases, including IBD [7]. For example, one study reported that the interaction between lncRNA H19 and the vitamin D receptor adversely affects intestinal epithelial barrier function in UC tissues [8]. Another study revealed differing expression levels of lncRNA during the remission and active stage of UC, thereby providing a research foundation for considering lncRNA as a potential biomarker for UC diagnosis [9]. DDX11-antisense RNA 1 (DDX11-AS1) is notably overexpressed in various malignant tumors, contributing to carcinogenesis by directly or indirectly modulating the expression of associated genes in cancers such as gastric, colorectal, bladder and breast cancers [10-13]. In a microarray analysis, Chen et al. identified 1211 lncRNAs in the plasma of CD patients, including upregulated DDX11-AS1 compared to a normal control group [14]. Despite this, there remains a relative paucity of studies addressing the expression trend of DDX11-AS1 in IBD, leaving its potential as a biomarker for IBD yet to be thoroughly explored.
In this study, we meticulously collected and analyzed the clinical data and gene expression levels from patients with IBD to assess the relationship between DDX11-AS1 and the severity of IBD. Additionally, we aimed to ascertain the potential of DDX11-AS1 as a diagnostic marker. Concurrently, we established an in vitro inflammation cell model to investigate the role and underlying mechanisms of DDX11-AS1 in IBD. Our findings aim to provide valuable data to support the clinical diagnosis and treatment strategies for IBD.
Material and methods
Study populations
This study comprised 58 patients diagnosed with IBD (including 39 with UC and 19 with CD) who were treated in the gastroenterology department. Additionally, 65 healthy individuals undergoing routine physical examinations during the same period were selected as the control group. The diagnosis of IBD adhered to the criteria established in the consensus on diagnosis and treatment formulated by the Gastroenterology Branch of the Chinese Medical Association (Beijing Conference) in 2018. This included assessments of clinical manifestations, complications, laboratory findings, colonoscopy and pathological manifestations, lesion scope, clinical type, and severity. Patients with infectious or other non-infectious enteritis, mental illness, other autoimmune diseases, and severe hepatic and renal insufficiency were systematically excluded from the study.
Fasting venous blood samples were collected from all subjects upon enrollment for subsequent laboratory examination. This study received approval from the Ethics Committee of Chenzhou No. 1 People’s Hospital, and all subjects have given informed consent.
Data collection
General information about the subjects was collected, including age, sex, height, weight, ethnicity, disease duration, smoking and drinking history. In addition, the laboratory parameters were recorded, including white blood cell count (WBC), hemoglobin, hematocrit (HCT), albumin (ALB), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP).
The assessment of disease activity and severity in UC was conducted using the Mayo score. A Mayo score of ≤ 2 indicates a state of remission, while scores ranging from 3 to 5 reflect mild activity. Moderate activity is denoted by scores between 6 and 10, and a score of 11 to 12 signifies severe activity [16]. For CD, the Crohn’s disease activity index (CDAI) score was used to evaluate the disease activity and severity. A score of less than 150 represents asymptomatic remission, 150-220 suggests mildly activity, 220-450 indicates moderately activity, and a score exceeding 450 represents severe activity [17].
Cell culture and transfection in vitro
Human normal colonic epithelial cells (FHC) were used as the experimental model in this study. The culture medium utilized was RPMI-1640, supplemented with 10% FBS, and maintained under conditions of 37oC in a humidified atmosphere comprising 5% CO2 and 95% air. Colitis cell models were constructed by treating FHC cells with different concentrations of lipopolysaccharide (LPS) (1, 3, 5, 10, and 15 ng/ml) for a duration of 6 h, referring to the published methods [18].
The siRNA sequence was designed and synthesized by GenePharma (Shanghai, China). The premix was prepared as follows: 100 µl of serum-free RPMI-1640 medium, 6 µl of Lipofectamine 3000, and 6 µl of siRNA (20 µM). After gently mixing the components, the premix was allowed to incubate at room temperature for 15 min. Subsequently, the incubated premix was added to the adherent cells, which were then cultured for 24 h.
Real time quantitative reverse-transcriptase PCR (RT-qPCR)
RNA was extracted from serum and cells using TRIzol reagent. RNA that met the requirements was immediately used for reverse transcription or stored at –80oC in an ultra- low temperature refrigerator. cDNA was synthesized with the HiScript cDNA Synthesis Kit (Vazyme). The amplification reaction was performed on ABI 8000 real-time quantitative PCR instrument. The total volume of the PCR reaction system was 20 µl, comprising 1 µl of cDNA template, 0.5 µl each of upstream and downstream primers, 10 µl of Ultra SYBR buffer, and 8 µl of RNase-free ddH2O. β-actin served as the internal reference gene, and the relative expression level of DDX11-AS1 was calculated using the 2–DDCt method.
In vitro cell viability assay
The cell counting kit-8 (CCK-8) assay was conducted for cell viability assessment. Briefly, cells (3 × 103/well) in the logarithmic growth stage with good growth status were inoculated into 96-well plates, with 5 compound pores per group, and cultured overnight in a cell incubator. Following cell adhesion, the relevant plasmids (si-NC and si-DDX11-AS1) were transfected and cultured for intervals of 24 h, 48 h and 72 h, respectively. Subsequently, LPS induction was performed for 6 h. Following this, 10 µl of CCK-8 solution was added to each well and incubated for an additional 2 h. The OD values at 450 nm were detected by a microplate reader, and the cell survival rate was calculated. Each experiment was performed in triplicate.
In vitro cell apoptosis assay
The cells in the logarithmic growth stage were prepared as a cell suspension (2 × 104/well), which was then inoculated into a 12-well plate. Following cell adhesion, transfection was performed, after which LPS-induced modeling was initiated. After these treatments, cells were harvested into a centrifuge tube, washed with PBS, and incubated in the dark for 2 h with Annexin V/FITC and PI dye. Finally, cell apoptosis was detected using flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
The supernatants from FHC cells across each group were collected, and the levels of tumor necrosis factor α (TNF-α), interleukin (IL)-6 and IL-8 were quantified using a commercial ELISA kit. In short, a standard solution, a concentrated washing solution and an antibody solution were prepared. Each well of the plates was designated as a blank, a standard or a sample to be tested. Following the addition of samples, the enzyme-labeled plate was covered with a film and incubated at 37oC for 2 h. The liquid in the plate was removed and washed with washing liquid four times. An antibody solution was then added to the well and incubated for another 1 h. After this incubation, 100 µl of chromogenic solution was added to each well, and the color was developed in the dark for 20 min. Finally, the OD value at 450 nm was detected using a microplate reader.
Luciferase reporter gene
Bioinformatics analysis focused on the regulatory role of DDX11-AS1 in targeting miR-2355-5p. To investigate this interaction, wild-type (WT) and mutant (MUT) luciferase reporter plasmids were constructed, followed by dual- luciferase reporting experiments to verify DDX11-AS1 as a ceRNA that adsorbs miR-2355-5p. The binding site predicted by the ENCORI database served as a template for amplification, leading to the insertion of these sequences into pmirGLO vectors to construct WT-DDX11-AS1 and MUT-DDX11-AS1. Concurrently, logarithmically growing cells were inoculated into 24-well plates, and these vectors were co-transfected with miR-2355-5p mimics and inhibitors. After 48 h of transfection, cells were collected for the detection of luciferase activity.
Data analysis
SPSS 22.0 and GraphPad Prism 7.0 statistical software were used for data analysis. The measurement data are represented as mean ± standard deviation, while the counting data are represented as n. Comparisons between two groups were conducted using an independent sample t-test, whereas multiple groups were compared by one-way ANOVA and the chi-square test. The diagnostic value of DDX11-AS1 in IBD was evaluated through receiver operating characteristic (ROC) analysis, evaluating the relative serum expression levels between the control group and IBD patients, as well as between UC patients and CD patients. The Pearson correlation coefficient method was employed to examine the relationship between CDAI and the Mayo score, in conjunction with the expression level of DDX11-AS1 (the value of the relative expression level of DDX11-AS1 in serum was used). A p value of < 0.05 was defined as statistically significant.
Results
Comparison of general information and clinical indicators between IBD patients and healthy people
As presented in Supplementary Table 1, this study encompassed a total of 123 participants, comprising 65 control individuals and 58 patients with IBD. There were no statistically significant differences in age, sex, body mass index (BMI), race, smoking and drinking history between the two groups (p > 0.05). In addition, the WBC levels in IBD were markedly elevated compared to those in the control group, while hemoglobin (Hb), HCT and ALB levels were significantly reduced (p < 0.001). Additionally, IBD patients also showed notable abnormalities in ESR and CRP levels.
Comparison of general information and clinical indicators between UC and CD patients
The cohort of 58 IBD patients was stratified into 39 individuals with UC and 19 individuals with CD based on the diagnostic results. As shown in Supplementary Table 2, the average age of patients with CD was lower than that of patients with UC (p < 0.05), while no significant difference in other indicators was observed between the two groups (p > 0.05). Furthermore, patients were classified according to CDAI and Mayo scores. Within the CD cohort, one patient achieved remission, three exhibited mild activity, fourteen presented with moderate activity, and one was classified as severely active. Among UC patients, three were in asymptomatic remission, 18 showed mild activity, 13 were moderately active, and five were severely active.
Expression level and diagnostic value of DDX11-AS1
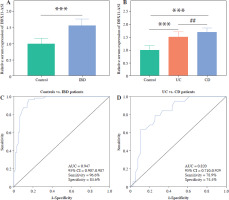
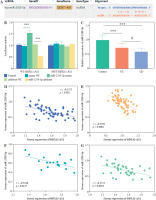
The results indicate significant up-regulation of serum DDX11-AS1 expression in IBD patients compared to the control group (Fig. 1A, p < 0.001). Upon classifying IBD patients, it was observed that DDX11-AS1 expression was elevated in UC, with an even greater increase noted in those with CD (Fig. 1B, p < 0.001). The ROC curve was constructed to assess the relative expression levels of DDX11-AS1 between the IBD group and the control group, revealing an AUC value of 0.947 (see Fig. 1C). Similarly, when evaluating the ROC curve using data from the UC and CD groups, the AUC was found to be 0.820 (see Fig. 1D). These findings illustrate that DDX11-AS1 possesses a distinct ability to discriminate between IBD patients and healthy individuals, demonstrating higher sensitivity and specificity in this regard.
Fig. 1
Serum expression levels of DDX11-AS1 and its potential as a diagnostic marker. A) Notably elevated expression of DDX11-AS1 in patients with inflammatory bowel disease (IBD). B) DDX11-AS1 expression increased in the UC cohort, with an even greater increase noted in the CD group. C) DDX11-AS1 effectively differentiates IBD patients from control populations, with the AUC value of the ROC curve being 0.947. D) DDX11-AS1 revealed a high diagnostic value in distinguishing between UC and CD, evidenced by an AUC value of 0.820. ***p < 0.001 compared with the control group, ##p < 0.01 compared with the UC group
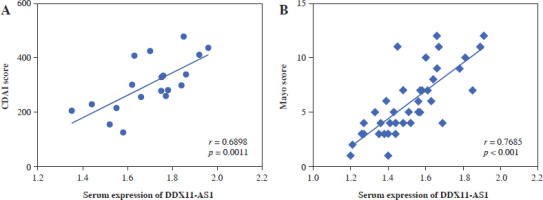
Correlation of DDX11-AS1 with disease severity score
The correlation was evaluated by the Pearson method. In CD patients, a positive correlation was observed between the CDAI score and the expression level of serum DDX11-AS1 (Fig. 2A, r = 0.6898, p = 0.0011). Similarly, in UC patients, a significant positive correlation was found between Mayo score and DDX11-AS1 expression level (Fig. 2B, r = 0.7685, p < 0.001).
Effects of DDX11-AS1 on FHC cell function and inflammation
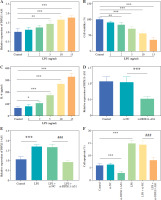
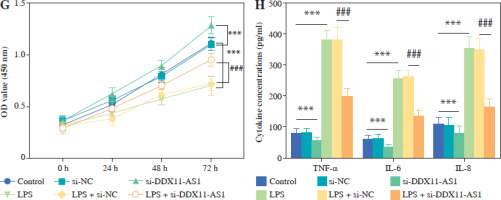
LPS was used to construct an in vitro inflammatory damage model using FHC cells. The expression level of DDX11-AS1 exhibited gradual up-regulation in response to increasing concentrations of LPS, with the most pronounced elevation occurring at concentrations of 10 and 15 ng/ml (Fig. 3A, p < 0.001). As shown in Figure 3B, the viability of FHC cells was inversely related to the concentration of LPS, demonstrating a steady decline as the concentration increased. Notably, at a concentration of 15 ng/ml, the viability of FHC cells was inhibited by over 50% (p < 0.001). Furthermore, the concentration of IL-6 increased concomitantly with the LPS concentration, indicating that LPS treatment effectively activated the cellular inflammatory response (Fig. 3C, p < 0.001). Considering the damage effect of a high concentration of LPS on FHC cells, 10 ng/ml was selected as the induction concentration for the cell model in this study, and this concentration was employed for subsequent experiments. The transfection efficiency of si-DDX11-AS1 was assessed, revealing that the transfection of si-DDX11-AS1 significantly down-regulated the intracellular expression of DDX11-AS1 (Fig. 3D, p < 0.001). Meanwhile, the downregulation of DDX11-AS1 markedly inhibited the LPS-induced elevation of DDX11-AS1 in FHC cells (Fig. 3E, p < 0.001). Further investigation of the effects of DDX11-AS1 on cell function showed that LPS induction notably enhanced apoptosis and decreased cell viability in FHC cells. However, transfection with si-DDX11-AS1 mitigated the adverse effects of LPS on cell function (Fig. 3F, G and Suppl. Fig. S1, p < 0.001). In terms of cellular inflammatory response, LPS treatment activated inflammation in FHC cells, as evidenced by the increased production of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-8. Conversely, pre-down-regulating DDX11-AS1 levels effectively attenuated LPS-induced inflammation (Fig. 3H, p < 0.001).
Fig. 3
Evaluation of LPS concentration and effects of DDX11-AS1 on cell function and inflammation. A) The expression level of DDX11-AS1 in FHC cells increased with rising LPS concentration. B) The viability of FHC cells diminished as LPS concentration increased. C) The levels of pro-inflammatory cytokine IL-6 escalated with the increase of LPS concentration. D) Transfection with si-DDX11-AS1 effectively down-regulated intracellular DDX11-AS1 expression in FHC cells. E) Transfection of si-DDX11-AS1 significantly mitigated the LPS-induced increase in DDX11-AS1 expression. F) Reducing DDX11-AS1 level markedly improved the promoting effect of LPS on apoptosis. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group, ###p < 0.001 compared with LPS + si-NC group G) Attenuation of DDX11-AS1 relieved the inhibitory effect of LPS on cell viability. H) Silencing DDX11- AS1 led to a reduction in LPS-induced inflammation, significantly decreasing the concentrations of TNF-α, IL-6, and IL-8. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group, ###p < 0.001 compared with LPS + si-NC group
MiR-2355-5p directly targets DDX11-AS1
The binding sites of DDX11-AS1 and miR-2355-5p, as predicted by the ENCORI database, are shown in Figure 4A. Figure 4B demonstrates that transfection of either miR-2355-5p mimic or miR-2355-5p inhibitor could reduce or enhance luciferase activity in the WT-DDX11 group (p < 0.001), whereas no such effect was observed in the MUT group. These results indicate that DDX11-AS1 is a direct target of miR-2355-5p. In clinical samples, the expression of miR-2355-5p in the serum of both UC and CD patients was decreased compared to healthy individuals, with the CD group exhibiting lower levels than those with UC (Fig. 4C, p < 0.05). Moreover, the Pearson correlation coefficient analysis revealed a significant negative correlation between the expression of miR-2355-5p and DDX11-AS1 in all IBD patients (Fig. 4D, r = –0.5373, p < 0.001). In the control population, a similar negative correlation was observed, further elucidating the targeted regulatory relationship between miR-2355-5p and DDX11-AS1 (Fig. 4E, r = –0.5392, p < 0.001). For CD patients, Figure 4F illustrates that the serum levels of miR-2355-5p were also negatively correlated with DDX11-AS1 (r = –0.5548, p = 0.0137). Furthermore, Figure 4G reveals a significant negative correlation between the expression of miR-2355-5p in UC patients and DDX11-AS1 (r = –0.4713, p = 0.0025).
Fig. 4
DDX11-AS1 directly targets miR-2355-5p. A) Proposed binding sequence between DDX11-AS1 and miR-2355-5p. B) The targeting relationship was validated through the luciferase reporter gene. C) Expression level of miR-2355-5p in the serum of clinical subjects. D) miR-2355-5p was negatively correlated with DDX11-AS1 in IBD patients. E) miR- 2355-5p was negatively correlated with DDX11-AS1 in the control population. F) Serum expression of miR-2355-5p was negatively correlated with serum expression of DDX11-AS1 in CD patients. ***p < 0.001 compared with control group. #p < 0.05 compared with UC group. G) Serum expression of miR-2355-5p was negatively correlated with serum expression of DDX11-AS1 in UC patients. ***p < 0.001 compared with control group. #p < 0.05 compared with UC group
Discussion
The pathogenesis of IBD remains incompletely understood, yet it has emerged as a global concern, characterized by high incidence rates, prolonged disease courses, frequent recurrences, and limited curability [20]. In the present clinical study involving 58 patients with IBD, we observed that DDX11-AS1 expression was significantly upregulated in the IBD cohort, with notably higher levels in the CD group compared to those with UC. ROC analysis demonstrated that DDX11-AS1 possesses high diagnostic accuracy for distinguishing IBD from control populations, as well as for differentiating UC from CD. In cell function studies, downregulation of DDX11-AS1 can significantly suppress the increase of DDX11-AS1 expression in LPS-induced FHC cells, achieved through the targeting of miR-2355-5p. This intervention also significantly reduced the apoptosis and inflammation induced by LPS. This analysis of IBD patient data revealed that the average age of onset of CD was younger than that for UC, with the majority of CD patients presenting between the ages of 25 and 29, indicating a relatively young demographic [21]. In contrast, the onset age for UC typically ranges from 21 to 40 years, with a higher incidence observed in males compared to females. This discrepancy may be related to lifestyle, smoking, drinking, environmental changes, and genetic predispositions [22]. Although this study did not reveal significant gender differences between UC and CD cases, both groups displayed a predominance of male patients. The assessment of IBD severity encompasses three aspects: 1) The impact of the disease on patients, including clinical symptoms and quality of life; 2) the extent and location of intestinal involvement; 3) disease activity, which provides a snapshot of the patient’s disease at a specific time (remission/low activity/moderate activity/high activity) [23]. For this investigation, Mayo and CDAI scores were employed to evaluate disease activity. The majority of the included patients with UC and CD exhibited mild to moderate disease severity, with 46.2% of cases categorized as mild UC and 33.3% as moderate UC. For CD, 15.8% were classified as mild, while a substantial 73.6% fell into the moderate category.
Emerging evidence suggests that lncRNA plays a pivotal role in the pathogenesis of various diseases. LncRNA exerts its biological functions by interactions with chromatin, transcription factors, RNA, and proteins. DDX11-AS1 has been implicated in the regulation of inflammation. In the context of IBD, while differential expression of lncRNA, including DDX11-AS1, has been reported in CD, studies specifically addressing its role in IBD remain limited. Much of the existing literature on DDX11-AS1 has concentrated on its function as an oncogene in cancers, with some investigations linking it to inflammation [24, 25]. In this study, it was observed that the serum expression levels of DDX11-AS1 in IBD patients were elevated compared to controls. Furthermore, within the IBD cohort, both CD and UC groups exhibited increased expression of DDX11-AS1; notably, the increase was more pronounced in the CD group than in the UC group. This finding aligns with the results of Chen et al., who reported up-regulation of DDX11-AS1 in the CD group through microarray analysis, although they did not examine the expression level of DDX11-AS1 in UC patients. In the current study, DDX11-AS1 expression was higher in CD patients compared to those with UC, and we speculated that the reasons for this result may be attributable to sample size and the stage of disease activity. Therefore, it is imperative to expand the sample size in further research to elucidate this phenomenon further. Additionally, this analysis showed a significant positive correlation between DDX11-AS1 levels and the Mayo and CDAI scores of IBD patients, indicating that the expression of DDX11-AS1 is closely related to the severity of IBD.
At present, it is widely acknowledged that abnormal intestinal mucosal function plays a crucial role in the pathogenesis of IBD and is influenced by a complex interplay of various factors. Research indicates a strong association between IBD and the development of digestive system tumors [26]. Therefore, the prevention and treatment of IBD are of great clinical significance for reducing the risk of digestive system tumors. Key inflammatory cytokines, including IL and TNF, are integral to the signal transduction pathways involved in the inflammatory response of IBD. Specifically, IL-6 is closely linked to stress, inflammatory infiltration and viral infection. Upon pathogen invasion, the inflammatory reaction is activated, with IL-6 serving as a principal mediator of this inflammatory cascade [27]. TNF-α plays a crucial role by inducing the chemotaxis of monocytes and neutrophils, enhancing the expression of endothelial cell adhesion molecules, and promoting the release of other pro-inflammatory cytokines, making it as a key cytokine in the pathogenesis of IBD [28]. Similarly, IL-8, a pro-inflammatory cytokine secreted by mononuclear macrophages, amplifies the local inflammatory response by mediating various signaling pathways that facilitate bactericidal action and cell damage, thus playing a key role in numerous inflammatory diseases, including IBD [29]. To explore the role of DDX11-AS1 in the inflammatory processes of IBD, this study builds on prior research by inducing FHC cells with LPS to construct an inflammatory model that simulates the inflammatory state of colorectal mucosa [30]. LPS, a specific component of the outer membrane of gram-negative bacteria, binds to corresponding proteins and antigens, activating NF-κB and subsequently interacting with Toll-like receptor 4 (TLR4) to promote the expression of pro-inflammatory factors such as IL-6. Notably, some studies have found LPS in the plasma of IBD patients, suggesting that impaired phagocytosis and diminished T-cell-dependent antibacterial activity in these individuals may explain its origin [31, 32]. Consequently, LPS is frequently utilized in vitro to simulate IBD status by intervening in normal colonic epithelial cells. In this study, in a certain concentration range, we observed that DDX11-AS1 increased after LPS treatment and showed a relative dependence on concentration. Based on the test results, 10 ng/ml was finally determined as the LPS-induced concentration. After transfection with si-DDX11-AS1, the expression of DDX11-AS1 was decreased after LPS induction. Meanwhile, the downregulation of DDX11-AS1 significantly increased the viability of FHC cells and inhibited apoptosis and inflammation. More and more studies have focused on the role of lncRNAs as ceRNAs in disease mechanisms. These lncRNAs can specifically adsorb some miRNAs like sponges and reduce the regulatory effect of miRNAs on mRNA[33]. For example, Ye et al. found that SNHG15 promoted the progression of liver cancer by negatively regulating miR-141-3p [34]. Yang et al. reported that lncRNA colorectal neoplasia differentially expressed (CRNDE) facilitated the apoptosis of colonic epithelial cells induced by dextran sulfate sodium by inhibiting the expression of miR-495 [35]. In this study, we employed StarbaseV2.0 to identify microRNA-2355-5p as a potential target of DDX11-AS1. MiR-2355-5p is known to be dysregulated in various human diseases. As an immunomodulation-related gene, its differential expression in tuberculosis suggests potential as a diagnostic marker [36]. Moreover, in ischemia-reperfusion injury, the upregulation of miR-2355-5p has been shown to alleviate the cardiac functional impairment and inflammation in an I/R-induced mouse model [37]. In this study, we confirmed the relationship between DDX11-AS1 and miR-2355-5p through luciferase reporter gene assays. Additionally, in clinical serum samples, the expression level of miR-2355-5p was down-regulated in the patient group, and the downregulation in the CD patient group was greater than that in the UC patient group. All these results can prove the relationship between DDX11-AS1 and miR-2355-5p.
The limitations of this study include a small sample size and a single study source, which may affect the reliability of the findings. To address these issues, future research should involve larger, multi-center prospective studies to confirm the current results. Additionally, the study only examined the effects of DDX11-AS1 on cell function and inflammation in vitro. Further investigation is needed to elucidate the underlying mechanisms, such as signaling pathways or molecular interactions through which DDX11-AS1 contributes to the development of IBD.
The study findings demonstrated that DDX11-AS1 expression was elevated in patients with IBD and correlated with disease severity. Additionally, down-regulating DDX11-AS1 in vitro mitigated LPS-induced inflammation, apoptosis, and promoted cell viability in FHC cells. These results suggest that DDX11-AS1 may serve as a potential biomarker and therapeutic target for IBD.