Purpose
In general, locally advanced cervical cancer is treated with external beam radiotherapy with concomitant cisplatin-based chemotherapy, followed by brachytherapy and occasionally adjuvant hysterectomy [1-4]. Brachy-therapy is an essential part of treatment of local cervical cancer, and is independently associated with improved cancer-specific and overall survival [5, 6], showing a loss of 12% of 4-year overall survival after a decline in brachytherapy use in the US [7, 8].
Few large studies have assessed prognostic factors in local cervical cancer. In 2016, Retro-EMBRACE showed that local control (LC) was correlated with radiotherapy doses that cover 90% of target volume (D90) and overall treatment time. A clinical target volume high risk adapted (CTV-T HRadapt) D90 of ≥ 85 Gy in seven weeks led to a 3-year LC rate of ≥ 94% for small residual tumors of < 20 cm3 [9]. In 2010, Touboul et al. demonstrated that the presence of residual disease in surgical pathology after neo-adjuvant chemoradiotherapy treatment was directly related to the risk of relapse and poor survival [10]. In parallel, a GYNECO 02 study [16] did not show a survival benefit of adjuvant hysterectomy in patients with pathological complete response; however, the study was prematurely stopped because of poor accrual. The role of adjuvant hysterectomy remains controversial owing to morbidity concerns and difficulties assessing survival benefit. Indeed, some studies suggest that increased survival depended on improving pathological complete response after neo-adjuvant treatment more than surgery alone [11, 12]. Magnetic resonance imaging (MRI), positron emission tomography-computed tomography (PET-CT), and now radiomics in response evaluation are the key for making the decision of surgery. In addition, FIGO (International Federation of Gynecology and Obstetrics) classification, nodal involvement, and tumor volume are independent prognostic factors, whereas age, anemia, histological type, and biological and molecular factors are still under discussion [13-15].
The objective of the present study was to investigate prognostic factors of LC and progression-free survival (PFS) of 3D pulsed-dose-rate utero-vaginal intracavitary brachytherapy in locally advanced cervical cancer, treated by chemoradiotherapy.
Material and methods
Study design
This was a retrospective single-institution cohort study. Institutional review board and ethical committee approved this retrospective study, and informed consent was waived for all subjects. A declaration to the French Authority Commission Nationale de l’Informatique et des Libertés was also issued.
Setting
Patients were recruited at the Institut de Cancérologie de Lorraine – Comprehensive Cancer Center by oncologists specialized in the management of gynecological cancers, between 2005 and 2015.
Participants
Eligibility criteria included a histologically proven local cervical cancer stages T1 and T2 without nodal or metastatic disease at CT scan. All histological types were accepted. The treatment was by curative intent, comprising external beam radiation therapy with concomitant chemotherapy or alone (in case of contraindication to chemotherapy), combined with image-guided adaptative brachytherapy based on MRI or CT results. Selected patients underwent adjuvant surgery at 6-8 weeks after the end of brachytherapy (total colpo-hysterectomy, and pelvic or para-aortic nodal disease in case of adenocarcinoma or for any residual tumor on clinical and MRI evaluation at 6 weeks post-brachytherapy). MRI and PET-CTs were performed at diagnosis. Exclusion criteria were previous treatments with neo-adjuvant or adjuvant chemotherapy, or interstitial brachytherapy. All patients at diagnosis underwent a gynecological examination with biopsies taken, under general anesthesia if needed, with cystoscopy and possible proctoscopy.
Intervention
Patients were treated with concomitant chemoradiotherapy, with 40 mg/m2 cisplatin weekly over 5 weeks. External beam radiation therapy was planned with 2 mm-slice CT with contrast (1.5 ml/kg) on a supine patient with full bladder. Pelvic external beam radiation therapy was delivered as 3D or intensity-modulated radiation therapy with a physical dose of 45 to 50.4 Gy in 25 to 28 fractions of 1.8 to 2 Gy, 5 times a week, using high energy photons (range, 10-25 MV). Pelvic or para-aortic nodal disease was treated with nodal boost (range, 5-15 Gy). Gross tumor volume (GTV) and clinical target volume (CTV) were contoured according to GEC-ESTRO guidelines. Gross tumor volume was not systematically delineated, in contrast to nodal GTV. Clinical target volume comprised the whole cervix and gross tumor volume, the whole uterus, parametria, and the upper part of the vagina (or more depending on its’ involvement). Nodal CTV involved bilateral external and internal iliac lymph node areas, ilio-obturator, and pre-sacral and common iliac areas. Treatment of para-aortic nodal disease was added in case of radiological involvement or notable pelvic nodal involvement. Planning target volume margin of 7 mm in all directions was generated from the nodal CTV and 10 mm from the tumor CTV. Organs at risk (OARs)’ constraints included a 30 Gy bladder volume ≤ 80%, a 40 Gy bladder volume ≤ 40%, and a bladder D2cc ≤ prescribed dose + 1 to 2 Gy. For the rectum and sigmoid, D2cc ≤ prescribed dose + 1 to 2 Gy, where dosimetry could be accepted if D2cc dose to the bladder/rectum was higher by maximum 1 to 2 Gy of prescribed dose. A 45 Gy femoral head volume ≤ 5% and 45 Gy bowel volume ≤ 200 cc (maximum, 250 cc) were aimed.
Image-guided adaptative brachytherapy was delivered in one pulsed-dose-rate application using a personalized vaginal mould applicator (intracavitary brachy-therapy). Target and OARs’ delineation were done on a treatment planning system (Oncentra®, Elekta, Stockholm, Sweden). High- and intermediate-risk clinical target volume (HR-CTV and IR-CTV, respectively), the bladder, rectum, and sigmoid colon were delineated according to the GEC-ESTRO guidelines. Then, dosimetry started with a 15 Gy prescription to point A (2 cm lateral to the central canal of the uterus, and 2 cm up from the mucous membrane of the lateral fornix, in the axis of the uterus) and 50 cGy per pulse. Optimization was performed graphically and manually, with dwell time modulation to control the delivered dose. Total planning aim dose for D90 HR-CTV was 85 Gy, and 60 Gy to D90 of IR-CTV. Dose constraints were the minimum dose in the most irradiated 2 cc (D2cc) of the bladder < 90 Gy, and D2cc of the rectum and sigmoid < 70 Gy. Dose-rate constraints to OARs were < 0.6 Gy/h. Total biologically equivalent doses in 2 Gy fractions (EQD2) of external beam radiation therapy plus brachytherapy were calculated by applying linear quadratic model with an α/β ratio of 10 for HR-CTV, 3 for OARs, and a half-time of repair of 1.5 h.
Follow-up
After treatment, patients were evaluated with a clinical examination every 4 months over 3 years, then every 6 months over 2 years, and annually thereafter. A pelvic MRI was done at 6-8 weeks after radiochemotherapy. In cases of a suspected relapse, biopsies were performed. CT scan and pelvis MRI were done annually, and PET-CT was optional.
Variables and definitions of clinical outcomes
Local control and PFS were calculated. Local relapses were defined as the period from the end of brachytherapy to any recurrence in HR-CTV or IR-CTV. Regional relapses were characterized as the time from the end of brachytherapy to any nodal recurrence in the pelvis or in the para-aortic areas. Other relapses were considered as metastatic failure, including carcinomatosis, mediastinal, or supra-clavicular nodal involvement. PFS was defined as the period from the end of brachytherapy to any failure. All failures were determined by combining clinical investigations (MRI, PET, and/or CT) and/or by pathological findings, and were classified as recurrent or persistent disease. Late toxicities in the rectum, bladder, and sigmoid were recorded according to the third version of common terminology criteria for adverse events (CTCAE v. 3), and were defined as clinical adverse event when appeared more than 3 months after the end of the treatment.
Predictors
We studied the prognostic factors related to cancer, patients, and treatment. For cancer, AJCC (American Joint Committee on Cancer) staging classification, nodal stage, histology and tumor volume before brachytherapy; for patient, age and WHO (World Health Organization) clinical progression scale; for treatment, methods of tumor’s and nodes radiotherapy (radiotherapy technique, total dose, fractionation, boost dose, radiotherapy field), characteristics of additional brachytherapy (total dose, HR-CTV and IR-CTV volumes, HR-CTV and IR-CTV D90, volume receiving a 60 Gy in brachytherapy, total reference air kerma, overall treatment time, concomitant chemotherapy, adjuvant hysterectomy, and pathological response to neo-adjuvant treatment).
Statistical methods
Quantitative parameters were described by median and interquartile range, and mean and standard deviation, qualitative parameters were defined by frequency and percentage. LC was determined using Fine-Gray model to account for competing risks of emergence of metastases or death, whatever the cause. PFS was determined using Kaplan-Meier method. Prognostic value of each factor was done using Fine-Gray model, and results were expressed as hazard ratio (HR) and 95% confidence interval (95% CI). Parameters with a p-value of less than 0.1 in bivariate analysis were examined using a full multivariate Fine-Gray model. This full multivariate model was then simplified with backward selection to avoid overfitting. The reduced final multivariate model was presented as adjusted HR and 95% CI. For PFS, the same process was performed using Cox proportional hazard model. All statistical analyses were done with SAS software, version 9.3 (SAS Institute Inc., Cary, NC 25513). P-values < 0.05 were considered statistically significant.
Results
This study retrospectively included 218 patients. The patient, tumor, and treatment characteristics are shown in Table 1. External beam radiation therapy comprised intensity-modulated radiation therapy in 47.3% of patients, whereas 84.4% of the patients (n = 184) underwent concomitant chemotherapy. The mean external beam radiation therapy dose was 45.3 ±1.7 Gy. Pelvic and para-aortic nodal diseases were treated with a mean dose of 53.6 ±5.9 Gy and 55.0 ±4.6 Gy, respectively. There were significant differences in age (p = 0.006) and completion of surgery (p < 0.001) in stages T1 and T2 patients.
Table 1
Characteristics of patients, tumors, and treatments
[i] Results presented with frequency and percentage [n (%)], or by median; mean ±standard deviation. SCC – squamous cell carcinoma, ADC – adenocarcinoma, PAN – para-aortic node, BT – brachytherapy, EBRT – external beam radiotherapy, 3D – conventional radiotherapy, IMRT – intensity-modulated radiotherapy, Gy – Gray, OTT – overall treatment time
Brachytherapy dosimetric parameters are presented in Table 2. All patients were treated with intracavitary brachytherapy and pulsed-dose-rate brachytherapy. The mean overall treatment time (OTT) was 55 ±12 days. There was a significant difference between AJCC stages T1 and T2 patients; stage II patients had higher OTT (p = 0.045), pulse number (p = 0.041), dose per pulse (p = 0.027), and D2cc to the bladder and rectum (p = 0.028 and p = 0.012, respectively). External radiotherapy and brachytherapy parameters according to surgery and pathological response are presented in Table 3. There was a significant difference between patients with complete vs. partial pathological response, concerning age (p < 0.001), chemotherapy (p = 0.006), volume receiving a 60 Gy in brachytherapy (p = 0.046), the rectum D2cc (p = 0.011), and OTT (p < 0.001).
Table 2
Brachytherapy dosimetric parameters
[i] Results presented with frequency and percentage [n (%)], or by median; mean ±standard deviation.
[ii] EQD2 – biologically equivalent dose in 2 Gy fraction, D2cc – the minimum dose in the most irradiated 2 cc, D90 – the dose that cover 90% of the target volume, HR-CTV – high-risk clinical target volume, IR-CTV – intermediate-risk clinical target volume, Gy – Gray, TRAK – total reference air kerma, OTT – overall treatment time
Table 3
Radiotherapy and brachytherapy parameters according to surgery and histological responses
[i] Results presented with frequency and percentage [n (%)], or by median; mean ±standard deviation.
[ii] EQD2 – biologically equivalent dose in 2 Gy fraction, D2cc – the minimum dose in the most irradiated 2 cc; D90 – the dose that cover 90% of the target volume, Gy – Gray, EBRT – external beam radiotherapy, TRAK – total reference air kerma, OTT – overall treatment time, HR-CTV – high-risk clinical target volume, IR-CTV – intermediate-risk clinical target volume
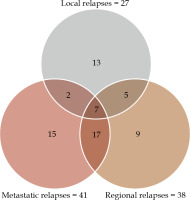
The median follow-up time was 4.2 years (interquartile range [IQR], 2.2-5.3 years). At the last evaluation, 152 (69.7%) patients were alive and 66 were dead. The median follow-up time of living patients was 4.7 years (IQR, 3.5-5.6 years). Local, regional, and metastatic relapses are presented in Figure 1. Overall survival was 83.9% (95% CI: 78.1-88.2) and 69.6% (95% CI: 62.5-75.5) at 2 and 5 years, respectively. LC was achieved in 87.8% (95% CI: 83.0-91.8) and 87.2% (95% CI: 82.3-91.3) of the patients at 2 and 5 years, respectively. Prognostic factors of local relapse are presented in Table 4. In bivariate analysis, surgery was a protective factor (hazard ratio [HR] = 0.37, 95% CI: 0.15-0.93, p = 0.035). Of note, there was no local relapse among the 42 patients with complete histological response (Figure 2B, p = 0.019). Among the operated patients, 45.8% with AJCC stage T1 and 46.5% with AJCC stage T2 tumors (p = 0.948) showed complete histological response (Table 1). In multivariate analysis, AJCC stage T2 (HR = 3.645, 95% CI: 1.27-10.46, p = 0.016) was the only independent prognostic factor associated with poorer LC. Figure 2A and B illustrates LC according to surgery and AJCC stages. PFS was achieved in 67.6% (95% CI: 60.9-73.4) and 57.4% (95% CI: 49.3-64.2) of the patients at 2 and 5 years, respectively. Prognostic factors of PFS are presented in Table 5. In multivariate analysis, para-aortic nodal disease (HR = 2.03, 95% CI: 1.16-3.57, p = 0.012), pathologic complete response (HR = 0.33, 95% CI: 0.15-0.73, p = 0.006), and IR-CTV volume > 60 cc (HR = 1.90, 95% CI: 1.22-2.98, p = 0.005) were independent prognostic factors associated with PFS. Figure 2C and D illustrates PFS according to surgery and AJCC stages.
Fig. 2
A-D) Local control and progression-free survival according to AJCC staging as well as surgery and pathological responses
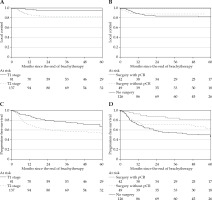
Table 4
Prognostic factors for local control
[i] HR (95% CI) – hazard ratio 95% confidence interval, TRAK – total reference air kerma, D2cc – the minimum dose in the most irradiated 2 cc, D90 – the dose that cover 90% of the target volume, HR-CTV – high-risk clinical target volume, IR-CTV – intermediate-risk clinical target volume, EBRT – external beam radiotherapy, IMRT – intensity-modulated radiotherapy, PAN – para-aortic node, N.S. – not significant
Table 5
Prognostic factors for progression-free survival
[i] HR (95% CI) – hazard ratio 95% confidence interval, TRAK – total reference air kerma, D2cc – the minimum dose in the most irradiated 2 cc, D90 – the dose that cover 90% of the target volume, HR-CTV – high-risk clinical target volume, IR-CTV – intermediate-risk clinical target volume, EBRT – external beam radiotherapy, IMRT – intensity-modulated radiotherapy, PAN – para-aortic node, N.S. – not significant
The incidences of severe (grades, 3-5) late toxicities are presented in Table 6, and there were no grade 5 (lethal) toxicities. At the end of follow-up, 12 patients suffered from at least one grade 3-4 toxicity, with a cumulative incidence at 5 years of 5.8% (95% CI: 3.2-9.6). At 5 years, the incidence of grade 3-4 bladder toxicity was reported as 4.3% (95% CI: 2.1-7.8), and both the rectum and sigmoid toxicities showed incidences of 2.0% (95% CI: 0.6-4.7). We found no significant difference regarding surgery. The mean doses to the bladder, rectum, and sigmoid of the entire cohort were 68 ±6.9 Gy, 53.7 ±5.0 Gy, and 54.2 ±4.9 Gy, respectively. No significant relationship between dose and toxicity was found (data not shown). Similarly, no difference in toxicity was found depending on a AJCC stage (data not shown). Cumulative incidence of grade 3-4 toxicity at 5 years for AJCC stage T1 cancer was 3.9% (95% CI: 1.0-10.0) (data not shown).
Table 6
Cumulative incidence of late adverse events grade 3-5 at 5 years according to surgery
Discussion
With a relatively long follow-up time, it is important to consider a real world data; therefore, we analyzed numerous patients treated with one type of applicator (a personalized vaginal mould applicator), which led to less variability in brachytherapy application and dosimetry. LC, PFS, and overall survival at five years were 87.2%, 57.4%, and 69.6%. We found that AJCC stage T2 patients had a poorer LC, with no significant relationship between LC and dosimetric parameters of brachytherapy, chemotherapy, surgery, external beam radiation therapy techniques, and overall treatment time. PFS had a better outcome with no para-aortic nodal disease involvement, pathologic complete response, and an IR-CTV volume of < 60 cc. Twelve patients (5.5%) presented ≥ grade 3 toxicity, but there was no significant difference between operated and non-operated patients.
In this study, the mean HR-CTV D90 of 72.1 Gy was lower than the 85 Gy GEC-ESTRO recommendation. Nevertheless, LC was satisfactory: for AJCC stage IB, 95% of patients had LC at 5 years with a HR-CTV D90 of 72.2 Gy. In comparison, in the RetroEMBRACE study, 98% of patients with AJCC stage IB tumors had LC at 5 years, with a HR-CTV D90 of 93 Gy. Bladder D2cc was lower in our study (66.2 Gy vs. 81 Gy), and with such a difference of D2cc to the bladder (and the rectum), a notable difference in terms of toxicity could be expected. Indeed, Mazeron et al. showed a significant correlation between D2cc to OARs and late rectal and urinary morbidity in patients treated with pulsed-dose-rate adaptive brachytherapy. In this paper, a bladder D2cc of 66 Gy suggested a 3-year, grade 2-4 morbidity-free survival in 94.5% of patients compared with 67.3% of patients treated with a D2cc > 80 Gy [16]. Therefore, dosage in patients with AJCC stage I tumors should be considered. In the present study, 83% of patients with AJCC stage II tumors achieved LC at 5 years, which was fewer than stage I patients, and patients in the RetroEMBRACE study, which reported LC at 5 years in 94% of stage IIA patients and 91% in stage IIB patients. These suggest that the higher HR-CTV doses in the RetroEMBRACE benefited AJCC stage II patients (mean HR-CTV D90 of 71.9 Gy vs. ≥ 88 Gy in the RetroEMBRACE study) [17, 18]. Thus, the lower LC in AJCC stage II patients in our study could be due to the difficulty of covering the target volume with only endocavitary brachytherapy techniques. In fact, AJCC stage T2 patients have a bigger tumor volume, especially in case of parametrial invasion (the largest proportion of our AJCC stage T2 patients). This higher dose is best achieved by adding interstitial needles in the parametrial region [19, 20] using UtrechtTM interstitial gynecological applicator and VeneziaTM interstitial gynecological applicator (Elekta), which is currently the practice in our institution for patients with parametrial involvement at diagnosis. Implanting interstitial needles is the best way to cover such a target region [21]. Lower doses delivered in this study may explain the absence of correlation between LC and dosimetric parameters of brachytherapy. No difference in OS between AJCC stage I and II patients were highlighted.
The role of adjuvant hysterectomy is still under investigation. With a high-risk of surgical complications in the irradiated area and non-established benefits, surgical intervention is not systematically utilized [22]. Our study showed that surgery is associated with LC outcome in univariate analysis (p = 0.035). However, this difference was not significant in multivariate analysis, suggesting the two groups were not well-balanced with respect to prognostic factors. In particular, concurrent chemotherapy was significantly less frequently used in non-operated patients (Table 3). In cases of pathologic complete response (42 patients), there was no local relapse, so multivariate analysis could not be used. For patients already in complete remission, it is unlikely that surgery would add benefit for LC. Like Touboul et al., our results suggest that pathologic complete response is associated with better LC and not surgery. For patients with residual disease, additional surgical procedure could in theory improve LC. However, no benefit of adjuvant hysterectomy in this study was found. The use of hysterectomy depends on clinical decision taken at the time of potential surgery, which is highly variable and depends on attending surgeons and institutional practices, which also changed between 2005 and 2015. Historically, favorable patients (< 50 years old and without nodal disease) were selected for surgery, and surgery was associated with better prognosis. Nowadays, the decision to operate is based on histological type (more frequently proposed for more adenocarcinoma cases with poorer prognoses) or for any residual tumor on clinical and MRI evaluation at 6 weeks post-brachytherapy. The best prognosis was associated with pathologic complete response, so higher doses of brachytherapy should be implemented to increase the rate of pathologic complete response in patients with stage II, while lower dose is sufficient for patients with stage I. For PFS, surgery was a significantly protective factor in univariate analysis. In multivariate analysis, pathologic complete response was associated with a significantly better PFS, lowering the risk by a third; although no benefit was seen in cases of pathological partial remission. An IR-CTV volume of > 60 cc, which corresponds to the tumor volume at diagnosis, also seems to be a significant prognosis factor for PFS in our study, as shown by Barillot et al. [23].
PAN involvement appears a significant prognostic factor for PFS. Nodal involvement is a well-known prognostic factor for survival even in early AJCC stage; AJCC stages T1b and T2a with lymph node involvement have a 78% 5-year survival, compared with 95% without nodal involvement [24]. For PFS, in a prospective PET-staged cohort, HRs for disease recurrence increased progressively in patients with nodal involvement based on the most metastatic nodal disease, compared with patients with no nodal involvement [25]. In the current study, para-aortic nodal disease was treated with extended field radiochemotherapy and nodal boost (at a mean dose of 55 Gy), and led to a 5-year PFS of 34.2% and of 61.5% in negative para-aortic nodal disease. Kidd et al. [25] reported a 3-year PFS of 34% in patients with AJCC stages T3 and T4 without nodal boost, whereas Castelnau-Marchand et al. [26] reported a 2-year PFS in 61.7% of patients, with a 60 Gy para-aortic nodal disease boost. Because few studies exist regarding treatment strategies for patients with para-aortic nodal disease, the literature shows it as tolerable technique, which improves loco-regional control and could lead to a survival benefit. Treatment intensification should be evaluated through increasing radiotherapy dose. OUTBACK trial of adjuvant chemotherapy is negative for a clinical benefit.
Our rate of severe toxicities is lower than in other studies, with 17 gastro-intestinal or urinary effects in 12 (5.5%) patients. By contrast, Castelnau-Marchand et al. reported a 6.6% rate of severe late effects [26-28]. The low-rate of severe late toxicity was to be expected, taking into account the low cumulative dose delivered to HR-CTV (72.1 Gy EQD2), resulting in low-dose to OARs. Still, it is surprising that there was no difference in the surgery vs. no surgery group, and that there was no dose dependence in toxicity.
Conclusions
Our results suggest that higher brachytherapy doses are necessary for LC in patients with AJCC stage T2 tumors, while it appears that the lower dose works better for patients with AJCC stage T1 tumors, for whom LC is already > 95%. According to these results, a change in our brachytherapy practice has already been implemented. The low-rate of severe toxicity has allowed higher doses to the target using parametrial interstitial needles, especially in AJCC stage T2 patients, with the aim to achieve better LC and PFS outcomes.