Introduction
Gastric adenocarcinoma is the second malignancy of the gastrointestinal tract and corresponds to 95% of all primary malignant tumours originated in this organ, and it has a poor prognosis; based on GLOBOCAN 2018 data, stomach cancer is the 5th most common neoplasm and the 3rd most deadly cancer, with an estimated 783,000 deaths in 2018 [1]. In recent years, the study of the molecular basis of neoplasms has begun, including gastric adenocarcinoma [2].
Microsatellite instability (MSI) occurs by defective repair of mismatching base pairs of methylated DNA (MMR), and it manifests as an abnormal length (increased or decreased) of the microsatellite repeats. The presence of MSI is a sign of deficiency in the repair of the mismatch of the DNA that in several cancers has shown prognostic impact [3, 4].
In gastric cancer, 2 systematic reviews demonstrated the better prognosis of MSI, but both showed high heterogeneity, the methodology for MSI detection was not standardized and was based on molecular analysis, the selection of patients varied across studies, and no neoadjuvant studies were included [4, 5]. MSI is detected only in cases of intestinal type, where it is statistically related to the progression of the disease [6]. MSI is present in 10–30% of cases [7].
The inactivation of the genes that repair replication errors leads (in most cases) to a loss of immunohistochemical (IHC) expression of the proteins encoded by these genes. In most of the studies and based on systematic reviews, the sensitivity and specificity of IHC for the identification of MSI are very high (> 90%), and the IHC expression analysis is a simple and inexpensive technique that is easy to perform in any laboratory where IHC staining is done routinely [8].
Our objective was to determine the 5-year overall survival (5-yr OS) of the MSI status demonstrated by IHC in gastric adenocarcinoma (both intestinal and diffuse cases), including cases with neoadjuvant therapy.
Material and methods
A cross-sectional study was carried out. All patients (> 18-years old) presented consecutively consulting for gastric adenocarcinoma between 2010 and 2013 were identified. We selected the cases in clinical stages I–III treated with D2 gastrectomy (median lymph node retrieval 46 nodes, range 29–115). We excluded stage IV patients due to its intrinsically poor prognosis, cases with carcinoma of the oesophagus or the oesophagogastric junction, cases that did not meet the pre-analytic requirements recommended for IHC studies (good fixation, use of buffered formalin, good preservation of paraffin blocks), and cases with absence of material for IHC.
The following data were collected from the files: age, sex, location of the lesion, presence of gastritis, infection by Helicobacter pylori, the presence of gastric atrophy, tumour type, HER2 status, histological grade, clinical stage, and overall survival. All the surgical samples were re-reviewed by 2 pathologists, and the IHC determination of the MMR proteins (anti-MLH1 [clone M1-Ventana, Oro Valley, AZ, US], anti-MSH2 [clone G219-1129 – Cellmarque, Rocklin, CA, US], anti-MSH6 [clone 44-Ventana], anti-PMS2 [clone EPR 3947 – Cellmarque]) was carried out as recommended in the consensus for the determination of MMR by IHC in colorectal cancer: the presence of nuclear expression in any percentage of the 4 proteins classified the case as microsatellite stable. Otherwise, the absence of at least one of the MMR proteins classify the case as MSI.
The primary outcome of this study was to determine if the presence of MSI in the gastric adenocarcinoma affects the 5-year OS of patients in I–III clinical stages. For all numerical variables we applied the Kolmogorov-Smirnov test to determine their normality. Data are represented as count and percentage for categorical variables and mean with standard deviation (SD) for numerical variables with parametric distribution, and we used medians and interquartile range (IQR) for non-parametric data. We used ANOVA or U Mann-Whitney testing for numerical variables and χ2 tests for categorical variables. We performed a univariate analysis with Kaplan-Meier curves to describe OS, and log-rank tests to compare the cumulative survival distributions between the groups. The Cox proportional hazard model for multivariate analysis was performed, adjusting the model for age, sex, and all variables with p < 0.10 in the univariate analysis. For all calculations, we set the statistical significance as a p-value < 0.05. We used SPSS 22.0 (SPSS, Chicago, IL, USA) to perform all statistics.
Results
Table 1 summarizes the variables according to the MSI status. From the 102 cases, 10 showed MSI (9.8%). The median age was 63 years (range 33–91 years), and 57.8% (59 cases) were men. The more prevalent site of occurrence was the antrum (46.1%) followed by the corpus (43.1%) and fundus (10.8%). From all cases, 78.4% presented in stage III. Regarding pathologic features, 47.1% were of diffuse type, 45.1% were of an intestinal type, and 7.8% were mixed type; whereas 74.5% were poorly differentiated (G3), 91.2% had chronic gastritis (36.3% caused by H. pylori and 11.8% had atrophy), and 36.3% showed intestinal metaplasia. Her 2 status was positive (score 3+) in 4 cases (3.9%) and negative in the remaining cases. From Table 1 is clear that MSI cases presented in earlier clinical stages (stages I–II) compared to MSS cases.
Table 1
Clinicopathologic features of 102 cases of gastric adenocarcinoma according their microsatellite instability status
Regarding outcomes, 14 (13.7%) cases recurred, 13 in the microsatellite stable group (p = 0.708), and 72 (70.6%) cases died, with a significant 5-yr OS between groups (Table 1, Fig. 1). In a stratified analysis by clinical stage, the difference in survival remained for patients with MSI.
Fig. 1
Kaplan-Meier survival curves of patients with gastric adenocarcinomas with determination of microsatellite instability by immunohistochemistry. Patients with microsatellite instability showed favourable overall survival
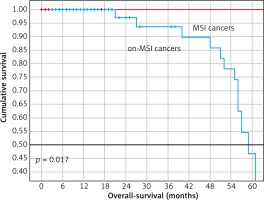
Table 2 shows the factors associated with survival in the 102 cases. The factors associated with poor survival were male sex, MSS cases, and being Her 2 negative. In a multivariate analysis, the MSI was independently associated with better survival (HR 0.209, 95% CI: 0.046–0.945, p = 0.042, Table 3).
Table 2
Factors associated with survival of 102 cases with gastric cancer treated by surgery from 2010 to 2013
Table 3
Multivariate analysis of factors associated with survival of 102 cases of gastric carcinoma
Discussion
In summary, of the 102 patients reviewed, 92 patients were MSS, and only 10 patients (9.8%) were MSI. MSI was associated with lower clinical stages, less recurrence, and better 5-year survival.
MMR deficiency noted in sporadic gastric cancers is caused by promoter methylation and the consequent suppression of transcription of MLH1. The MMR deficiency results in the accumulation of frameshift mutations of many target genes that have repetitive sequences in their coding region [9]. As a result, MSI gastric cancers follow a unique, multistep carcinogenesis pathway. It has been published that MSI gastric cancers are thought to have different clinicopathological features compared to other groups that support the “classic” or “chromosomal instability” pathway, an association with female sex, older age, intestinal type, mid/lower gastric location, lack of lymph node metastasis, and TNM stages I–II [4]. We did not find any clinicopathologic differences between groups except clinical stages I–II, according to data from other published studies (Fang). A plausible explanation is that several of the published series focused on intestinal-type adenocarcinomas, whereas we included all gastric carcinoma subtypes.
There is a prognosis discrepancy of MSI in gastric carcinoma. A better prognosis of MSI has been reported in some studies [10–15], especially in intestinal adenocarcinomas and those with distal location [16, 17], but not in others [18, 19]. This discrepancy could be explained by different incidence (8.2–37%), use of different MSI definitions, determination of MSI status by tissue microarrays, or the limited numbers of cases (11–83 cases) of the previous studies. We found that the MSI cases had a better prognosis (p = 0.042).
Finally, in this study, we found that the frequency of MSI-H gastric cancers was 9.8%. This frequency is in agreement with the data of previous studies – from 8.2% to 9.6% [18, 20, 21].
Some limitations exist in the present study: this was a single-centre study, and some of the groups had a small number of cases (particularly the Her 2-positive cases). Among the strengths, our research is based on a public high-volume cancer centre; the sample represents Latin patients treated with potentially curative intent by high-volume surgeons were standard D2-gastrectomy was performed, the pathologic evaluation is well standardized, and the period of patients’ recruitment is short, which makes possible a standardized and homogeneous criteria for treatment.








