Purpose
Cervical cancer is the most common gynecological malignancy, representing the fourth most common malignancy among women. Radiotherapy has been used for more than a century, with the first documented application of radium (Ra) performed in 1903, and the first cured patient reported in 1913. Radiation therapy is currently a method of choice in patients with early stage disease (I-IIA) and represents, in combination with chemotherapy, a standard treatment option for patients with locally advanced tumors.
Brachytherapy is an integral component of cervical cancer radiotherapy, significantly improving overall survival [1]. With technological progress and development of computed tomography/magnetic resonance (CT/MR) compatible applicators, 3D imaging was gradually implemented to brachytherapy treatment planning. The use of CT and MR images allow to determine the exact target volume and contours of organs at risk (bladder, rectum, and sigmoid), followed with targeted plan optimization using 3D images [2,3,4,5,6,7,8,9,10]. Shin reported the effect of imaging methods on final plan quality, aiming at conformity of targeted volume and contouring of organs at risk [7]. The positive effect of 3D brachytherapy using magnetic resonance on locoregional recurrence and overall survival was recently published by Pötter [3,11].
Gynecological GEC-ESTRO working group published the 3D brachytherapy recommendations related to determination of targeted volumes, planning concept based on the dose volume histogram parameters (DVH), reconstruction of applicators, and basic principles and parameters for MR imaging [12,13,14,15]. With these guidelines, the Gynecological GEC-ESTRO working group presented a common concept and terminology for different clinical approaches and sources used in radiation. Magnetic resonance allows the delineation of the area with macroscopic tumor (gross tumor volume – GTV). The working group also recommends different clinical target volumes for areas with high-risk and intermediate-risk of recurrence (high-risk clinical target volume – HRCTV, intermediate-risk clinical target volume – IRCTV), and recommends also reporting the minimum applied dose in 90% and 100% (D90, D100) in the case of clinical target volume and minimum dosage applied for 0.1 cm3, 1 cm3, and 2 cm3 (D0.1cc, D1cc, and D2cc) for organs at risk.
The undeniable clinical benefit is associated with more time-demanding process of contouring and planning, which leads to extended interval between imaging (after insertion of applicators) and radiation itself.
The purpose of this study was to evaluate the outcome and clinical influence of the planning time factor on resulting distribution dose.
Material and methods
Dynamic changes of topography of the small pelvis during brachytherapy planning in 10 patients who underwent chemoradiotherapy for locally advanced cervical cancer (cT2b cN0-1) were evaluated.
Patients underwent pelvic external irradiation with intensity modulated therapy (IMRT) 45 Gy per 1.8 Gy in 25 fractions, with concurrent weekly administration of cisplatin (40 mg/m2). The treatment was subsequently continued with 3D-based brachytherapy with prescribed dose of 4 × 7 Gy for minimal coverage of 90% high-risk clinical target volume (D90 HRCTV), twice a week using Utrecht CT/MR compatible applicator (Nucletron; Elekta, Stockholm, Sweden). During the procedure, the bladder was routinely emptied and instilled with 50 ml of saline to maintain the anatomical geometry. All women were asked to follow dietary protocol to ensure empty rectum. Immediately after applicators insertion in operating room, patients were transported to brachytherapy room, where the CT acquisition was performed by 3 mm slice thickness using GE Healthcare LightSpeed [16]. Complete CT image datasets for brachytherapy were transferred to Oncentra treatment planning system version 4.3 (Elekta; Nucletron, Stockholm, Sweden) for contouring and planning. All patients were contoured by a single physician (Z.V.) and planned by a single physicist (K.S.).
The planning CT scan was used for creating a regular 3D plan using the Gynecological GEC-ESTRO working group recommendations. Aside from recommended dosage of 4 × 7 Gy for D90 HRCTV, the DVH constrains for rectum, bladder, and sigmoid were also used. The limit summary equivalent dose in 2 Gy fraction for 2cc (EQD2 D2cc) for bladder was 90 Gy, and for sigmoid and rectum 75 Gy. High-dose-rate brachytherapy was performed using 192Ir remote afterloading system (MicroSelectron, Elekta, Stockholm, Sweden), with the patients still lying on the CT table to minimize motion of applicators.
A second CT scan (confirmation CT) was performed in the first and third fraction of brachytherapy, after creating the plan just before the irradiation. After irradiation, the applicators were removed.
In a confirmatory CT scan, the clinical target volumes and high-risk organs were contoured. Applicators were reconstructed and this plan consisted of stepping source positions based on the original plan. Twenty plans based on planning CT scans and 20 confirmation plans based on confirmatory CT scans were compared in terms of changes of volumes and differences in doses to high-risk organs. At the same time, the relation between planning time and dose to organs at risk were evaluated. Plans created in less than 54 minutes versus plans generated in 54 minutes or more were compared. To evaluate the doses for bladder, sigmoid, and rectum, the Gynecological (GYN) GEC-ESTRO working group doses parameters for 0.1 cm3, 1 cm3, and 2 cm3 (D0.1cc, D1cc, and D2cc) were used. The difference of dose between confirmation CT and planning CT for each dose parameter was assessed (ΔD), and the correlation coefficient was used to evaluate this association. Additionally, the correlation coefficient was used to express a linear relationship between two random variables X, Y. The sample correlation coefficient RX,Y of random variables X, Y is a number defined by the relation:
Results
The median planning time performing planning CT scan and confirmatory CT scan before the irradiation was 54 minutes (range, 36-64 minutes). In 10 patients, planning time was less than 54 minutes and in 10 patients was 54 minutes or more.
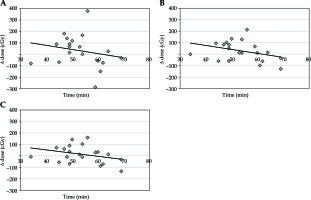
The median volume of the bladder in the planning CT scan was 119.6 cm3 and 141.0 cm3 in the confirmatory CT. The absolute median change of volume was 32.1 cm3 (range, 1.6-108.6 cm3). This change represents a difference of 27.9% from the original volume (range, 0.96-155.5%). This difference led to an increased median dose for delta D0.1cc by 46.7 cGy, for delta D1cc by 59.2 cGy, and for delta D2cc by 44.7 cGy per each single fraction (Table 1, Figure 1A-C).
Table 1
The difference of dose (ΔD) between confirmation CT and planning CT for each dose parameter (D0.1cc, D1cc, D2cc) for organs at risk
Fig. 1
A) Time vs. change of dose for D0.1cc bladder; B) Time vs. change of dose for D1cc bladder; C) Time vs. change of dose for D2cc bladder
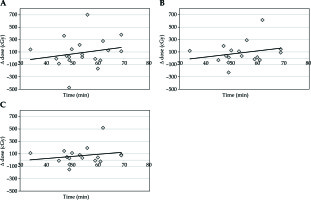
The median volume of rectum in the planning CT scan was 45.5 cm3 and 41.6 cm3 in the confirmatory CT. The absolute median change was 5.6 cm3 (range, 0.4-61.8 cm3), with a difference of 14.4% from the original volume. This volume change led to decreased dose per each fraction for D0.1cc of 7.1 cGy, for D1cc of 3.5 cGy, and for D2cc of 4.8 cGy (Table 1, Figure 2A-C).
Fig. 2
A) Time vs. change of dose for D0.1cc rectum; B) Time vs. change of dose for D1cc rectum; C) Time vs. change of dose for D2cc rectum
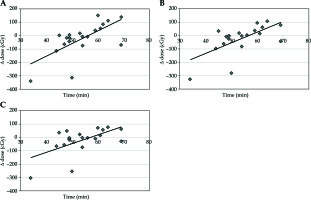
The median volume of sigmoid in the planning CT scan was 33.7 cm3 and 32.1 cm3 in the confirmatory CT. The median absolute volume change was 8.4 cm3 (range, 0.2-74.1 cm3), corresponding to 26% (range, 0.8-428.7%). The dose for sigmoid for D0.1cc was increased per each separate fraction by 25.7 cGy for D0.1cc, 11.8 cGy for D1cc, and 10.0 cGy for D2cc (Table 1, Figure 3A-C).
Fig. 3
A) Time vs. change of dose for D0.1cc sigmoid; B) Time vs. change of dose for D1cc sigmoid; C) Time vs. change of dose for D2cc sigmoid
The relation between the planning time and the delta dose was evaluated. No significant changes were noted for sigmoid (R for D0.1cc was –0.2267, for D1cc –0.3482, and for D2cc –0.3184) and bladder (R for D0.1cc was 0.2067, for D1cc was 0.2637, and for D2cc was 0.2398). However, a statistically significant association between the planning time and delta dose was detected in all observed volumes for the rectum. The longer the planning, the higher the dose. The correlation coefficient for D0.1cc was 0.6715 (p = 0.0061), for D1cc was 0.6404 (p = 0.011), and for D2cc was 0.5891 (p = 0.0197).
The median absolute volume change for bladder, rectum, and sigmoid in plans created less vs. more than 54 minutes were 24.24 cm3 vs. 32.26 cm3, 6.25 cm3 vs. 4.62 cm3, and 8.44 cm3 vs. 7.9 cm3, respectively. The median delta dose between the original plans and actual plans for patients with a planning time more than 54 minutes and for women with shorter period of planning time than median was increased for rectum and bladder by 99 cGy and 91.9 cGy for D0.1cc, 61 cGy and 52.1 cGy for D1cc, and 29.8 cGy and 44.9 cGy for D2cc per each fraction. On the contrary, a decrease of dose for sigmoid by 133.96 cGy, 70.3 cGy, and 48.6 cGy, respectively, was observed.
Discussion
Image-guided CT/MR brachytherapy significantly improves therapeutic results for patients with locally advanced cervical cancer [11,16]. The Gynecological GEC-ESTRO working group has initiated the multicenter observational EMBRACE studies (“Image-guided intensity modulated external beam chemoradiotherapy” and “MRI-based adaptive brachytherapy in locally advanced cervical cancer”), bringing new data regarding locoregional control, nodal control, overall survival, morbidity, quality of life, and prognostic and predictive parameters [17,18,19,20].
The 3D treatment planning time is more time-consuming than regular 2D planning. Based on the changes of small pelvis topography in time, the focus of the present study was to determine whether a longer treatment planning time can have dosimetric consequences with regards to the dose for organs at risk [21,22]. The most significant changes observed were for the rectum. With the prolongation of planning time, the rectal volume increased, leading to a significant dose increase. The median delta doses between the original plans and actual plans were compared for women with planning time longer than median (54 minutes) and patients with shorter planning time. A dose increase of 99 cGy for D0.1cc, 61 cGy for D1cc, and 29.8 cGy for D2cc per each fraction was observed in women with planning time longer than 54 minutes. In comparison with the median original planning dose, this means a possible increase by 22.3% for D0.1cc, 18.4% for D1cc, and 9.6% for D2cc. We believe that most important reasons for the difference in planning times was difficulties in some of the plans with respect to the small pelvis topography and due to time-consuming manual optimization used.
Interestingly, correlation between change of volume and change of the dose was confirmed only for rectum. It is possible that changes in volume in sigmoid and bladder are more variable, while increasing the volume of rectum during planning process is more associated with the reduction of distance between rectum wall and applicators.
To the best of our knowledge, this is the first study focusing on the impact of planning time on the actual dose to organ at risk. Based on the present results, it seems that longer period of high-dose-rate (HDR) brachytherapy planning time can lead to incorrect estimation of dose distribution, principally to the rectum. The actual dose distribution does not correspond to the dose distribution at planning CT, because of the temporal changes in pelvic topography. It is therefore imperative to decrease the planning time.
The small number of patients is a significant limitation of the present study. In order to fully confirm current observations, a larger patient cohort is needed. However, despite this limitation, the present data support the conclusion that the planning time affects the real dose distribution. Based on these findings, a strive for a minimal delay between application and radiotherapy seems to be appropriate.



