Purpose
Prostate cancer is one of the most common cancer types in men. Currently, there are several effective treatment options available, including radiation therapy, but the ideal radiation dose is still unknown. Nevertheless, an increasing number of published data on prostate cancer demonstrated significant dose escalation as the most efficient treatment strategy, preventing patients from local tumor recurrence [1-3].
Interstitial brachytherapy (BT) is a well-established part of radiation treatment [4, 5]. Due to steep dose gradient of BT, radiation dose within the prostate gland can be escalated while sparing surrounding organs at risk (OARs).
Several studies showed a significant benefit for biochemical recurrence-free survival (bRFS) as well as for prostate cancer-specific mortality when combining external beam radiotherapy (EBRT) and BT, compared with EBRT only [6-10]. In a randomized phase III trial, Hoskin et al. showed a significantly improved recurrence-free survival (RFS) in patients receiving a B-boost compared with patients receiving EBRT only [11]. Toxicity rates were similar in both the groups. These results support focusing on higher radiation doses within target volume. On the other hand, late toxicities need to be considered since radiotherapy of prostate cancer is curative for most patients. When using BT as a boost for prostate cancer, serious complications occur generally rare [12, 13]. However, with dose escalation, toxicity in genitourinary tract (GU toxicity) can increase, as reported in the ASCENDE-RT trial [14]. In this trial, patients were randomized to receive either EBRT alone or EBRT and BT boost [14]. After 10 and 15 years of follow-up, patients treated with EBRT and BT boost showed an significant increasing improvement in biochemical progression-free survival. Moreover, GU side effects increased compared with patients receiving EBRT only [15, 16]. This confirms the importance of long-term follow-up when discussing treatment strategies for prostate cancer patients.
In combining EBRT and BT, dose escalation is feasible and very effective, but needs further detailed evaluation. In the present analysis, we reported long-term results of our protocol-based standardized treatment using EBRT and BT boost.
Material and methods
Patients with non-metastatic prostate cancer treated in our hospital from 2008 to 2016 using BT boost post-EBRT outside other studies were included. Inclusion criteria for the present analysis were histologically confirmed non-metastatic prostate cancer and available basic data, such as age, tumor stage, prostate specific antigen (PSA) value, risk group according to D’Amico classification [17], lymph node involvement, hormonal therapy received, EBRT and BT treatment details, and follow-up data on PSA value, local-regional tumor status, metastatic disease, and treatment-related side effects. All patients were informed about treatment details and provided written consent.
Patients were treated with a BT boost after completion of EBRT. Whether regional lymph node areas were irradiated with EBRT was determined by the risk of tumor invasion according to the Yale-formula [18]. Generally, whole pelvis irradiation was performed if the risk of lymph node involvement exceeded 15%. Clinical target volume (CTV) for EBRT was defined as the entire prostate gland and seminal vesicles, with safety margins of 5-10 mm in all directions, based on a planning from computed tomography scan. For high-risk patients, the internal and common iliac nodes areas were additionally included in CTV, with a safety margin of 7 mm. An extra margin of 0.5 cm up to 1.5 cm was added to CTV to obtain planning target volume (PTV). General dose schedule for EBRT was 1.8 Gy per fraction, delivered in 5 days per week, up to a total physical dose of 50.4 Gy.
Following EBRT, boost irradiation was performed using temporary interstitial BT with an iridium-192 afterloader. BT technique was utilized according to Stromberg et al. [19], as previously reported in detail [20]. Briefly, under high-resolution trans-rectal ultrasound (TRUS) guidance, titanium needles of 200 mm length were inserted trans-perineal into the prostate. Fixation of the needles was supported by a special template sewn to the perineal skin. BT target volume was limited to the prostate gland as defined by TRUS. No additional safety margins were applied. Dose distribution was calculated using a geometric and graphical forward planning approach, considering dose constraints for the rectum and bladder as 75 Gy and 85 Gy (EQD2α/β = 3, including EBRT dose), respectively. BT was delivered either as high-dose-rate brachytherapy (HDR-BT) with 2 × 9 Gy or 2 × 9.5 Gy schedule, up to a total dose of 18 Gy or 19 Gy (EQD2α/β = 3 = 43.2-47.5 Gy), or as pulsed-dose-rate (PDR)-BT with a pulse dose of 0.7 Gy per hour, for 24 hours per day, up to a total dose of 35 Gy (EQD2α/β = 3 = 45.13 Gy) [20].
At each follow-up visit, PSA level, tumor status, treatment-related toxicity according to the LENT-SOMA scale and RTOG grading, and physical health were documented. In the past, the LENT-SOMA scale was used more frequently in our clinic for follow-up documentation. Therefore, the analysis of toxicity outcome was based on the LENT-SOMA scale. Both cumulative incidence and prevalence at 5 years were evaluated to distinguish the difference of transient and long-term toxicity. PSA recurrence was defined as per Phoenix definition, which implies a rise of PSA level at least 2 ng/ml above the lowest measured PSA level after treatment (“nadir”) [21]. Local recurrence was either histologically confirmed or diagnosed with prostate-specific membrane antigen positron emission computer tomography (PSMA PET-CT).
Local recurrence rate (LRR), bRFS, tumor-specific survival, and overall survival (OS) rates were estimated using Kaplan-Meier method. Log-rank test (Mantel-Cox test) was applied to determine statistical significance of differences between the groups. Toxicity was analyzed using chi-squared test and Fisher exact test. Statistical analysis was performed with IBM SPSS Statistics (version 28.0.0.0; IBM, USA).
Results
Patient characteristics
From 2008 to 2016, 115 patients with non-metastatic prostate cancer were treated with EBRT and BT boost, and were included in this analysis (Table 1). The median age was 72 years (range, 48-83 years). The median initial PSA value was 12.2 ng/ml (range, 2.4-288.0 ng/ml). 78/115 patients (68%) were included in the high-risk group, according to D’Amico [17]. In 35/115 patients (30%), the criteria for the D’Amico intermediate-risk group were met, while 2 patients (2%) were included in the low-risk group. In 7 patients (6%), regional lymph node metastases were initially diagnosed, while three patients (3%) were known to be oligo-metastatic at the time of the treatment. Hormonal therapy was already initiated at the time of radiation therapy in 57 patients (50%).
Table 1
Patient characteristics (n = 115)
All patients were first treated with EBRT. Pelvic lymph nodes were irradiated in 67/115 (58%) patients, and 48/115 (42%) patients received irradiation limited to the prostate and seminal vesicles. Following EBRT, 32/115 (28%) patients received HDR-BT, and 83/115 (72%) patients received PDR-BT. The median follow-up time was 89 months (range, 9-159 months).
Efficacy
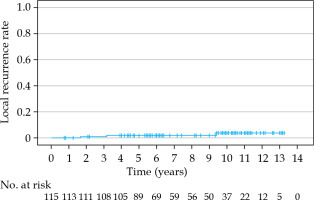
The estimated cumulative LRR at 5, 7, and 10 years was 1.8%, 1.8%, and 3.8%, respectively (Figure 1, Table 2). Local recurrence was detected in three patients (3/115, 3%), all of whom were initially classified as high-risk, according to D’Amico. In one of these three patients, local recurrence was histologically confirmed, and the patient received systemic palliative therapy. Another patient was diagnosed with local recurrence shown on PSMA PET-CT scan; he was successfully treated with prostate salvage brachytherapy, stereotactic radiotherapy for detected lymph nodes, and hormonal therapy.
Table 2
Outcomes
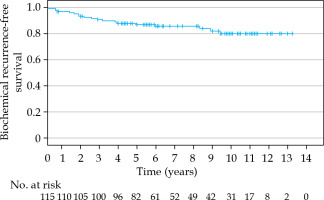
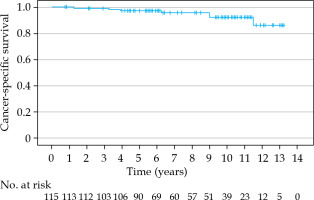
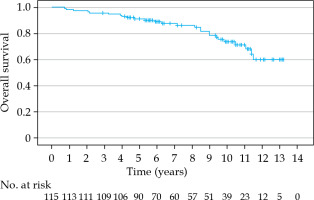
The estimated 5-, 7-, and 10-year bRFS rates were 86.5%, 85.2%, and 79.5%, respectively (Figure 2). In 6 patients (6/19, 32%), the recurrence was detected only biochemically without any clinical correlation with PSA elevation. Bone metastasis and nodal recurrence were the most common sites of recurrence. Patients with an initial PSA of 20 ng/ml or more had a significantly worse bRFS (p = 0.010). Also, the D’Amico high-risk group had a significantly worse bRFS (p = 0.002). Hormonal therapy treatment did not significantly influence bRFS rate (p = 0.097). The cancer-specific survival (CSS) at 5, 7, and 10 years was 97.3%, 97.3%, and 92.2%, respectively (Figure 3). The 5-, 7-, and 10-year OS rates were 91.2%, 88.9%, and 73.6%, respectively (Figure 4).
Comparing patients treated with HDR (32/115, 28%) and those treated with PDR (32/115, 72%), there were no significant differences for LRR (p = 0.327), bRFS (p = 0.141), CSS (p = 0.193), and OS (p = 0.268).
The median prostate volume was 30.9 cm3 (range, 9-78.7 cm3). For patients with a prostate volume of 50 cm3 or larger (16/114, 14%), no significant difference for LRR (p = 0.523), bRFS (p = 0.383), CSS (p = 0.424), and OS (p = 0.117) was seen compared with patients with a prostate volume of less than 50 cm3 (98/114, 86%).
Late side effects
At five years, the cumulative incidence of side effects according to the LENT-SOMA scale of any grade was 50% (Table 3). The most common late side effect of any grade was impotence (30%), followed by incontinence, urethritis (17%), and proctitis (14%). At five years, the cumulative incidence of side effects of at least grade 3 was 16% (18/115 patients). One patient developed a rectal ulcer and needed surgical treatment. On the other hand, the prevalence of side effects of any grade at 5 years after treatment was 18% (14/80), with mainly genitourinary side effects. After 5 years, the prevalence of side effects of at least grade 3 was 4% (3/80), with all 3 patients reporting incontinence. Side effects at any time were prevalent in both the HDR (18/38, 47%) and PDR (38/83, 46%) treatment groups, with no statistically significant difference (p = 0.314). Regarding the prevalence at 5 years, no statistically significant difference in-between was seen in the treatment groups concerning any grade of side effects (p = 0.613) nor side effects ≥ 3 (p = 0.540). Patients with a prostate volume of 50 cm3 or larger did not report significantly more side effects (p = 0.077). Regarding the specific side effects, only the prevalence of urethritis at 5 years post-treatment was significantly increased in patients with a prostate volume of 50 cm3 or larger (p = 0.035).
Table 3
Treatment toxicity according to the LENT-SOMA scale
Discussion
Close relation between tumor control probability and total radiation dose is a well-known fact in prostate cancer radiation treatment [1, 2]. Our reported efficacy of brachytherapy as dose escalation with a 7-year cumulative recurrence rate of only 1.8% and a 7-year cancer-specific survival of 97.3% using total doses (EBRT + BT) up to 95 Gy (EQD2α/β = 3), is undoubtedly high. At the same time, side effects were very limited. These results are consistent with a 10-year data of the TROG 03.04 RADAR trial [22, 23]. In this trial, more than 1,000 patients with locally advanced prostate cancer were treated in 23 centers. Sub-group analysis of 237 patients treated with BT boost showed a significant reduction of risk for local progression, prostate cancer-specific mortality, and distant metastasis, regardless of anti-hormonal therapy duration. Local progression within the BT boost group was only 2.2% at 10 years. Importantly, the sub-groups in this study were not balanced regarding initial-risk state. In the sub-group of patients receiving BT boost, the rate of high-risk patients (85.7%) was much higher than in the EBRT group [22, 24]. An initial T3-4 tumor stage was prevalent in 64.1% of BT boost patients vs. 24.8-31.7% of EBRT patients. In the RADAR trial, even though the applied total radiation dose of EBRT without BT boost was below current standards, these results show the importance of dose escalation strategies. The results of the present study are very similar. Within 7 years, we observed a local recurrence rate in only 1.8% of our patients, which is in accordance with the results of the RADAR trial.
The benefit of dose escalation was also demonstrated by Hoskin et al. In their prospective, randomized phase III trial, 218 patients were treated either with EBRT alone or combined with BT boost [11]. EBRT alone consisted of 20 × 2.75 Gy (EQD2α/β = 3 = 63.25 Gy) compared with 13 × 2.75 Gy EBRT followed by 2 × 8.5 Gy HDR boost (EQD2α/β = 3 = 80.21 Gy) for the BT boost cohort. The bRFS rate according to the Phoenix definition was 75% at 5 years for the boost arm, as opposed to 61% in the EBRT alone arm. In the 12-year update of the study, an improvement of 21% for the boost arm was confirmed [25]. Our results (7-year bRFS rate of 85.2%) are comparable, even slightly better. Other retrospective studies have reached similar conclusions. For example, Martell et al. found a bRFS rate of 91% at 5 years in 518 patients who received EBRT and BT boost after being diagnosed with intermediate-risk prostate cancer [26]. The median age in that retrospective analysis was 67 years, as opposed to 72 years in our cohort. The bRFS rate at 5 years was slightly less in our cohort, with 86.5%. The reason is most likely the inclusion of high-risk patients in our analysis.
Besides tumor control, patients are expected to experience as little side effects as possible. Considering excellent tumor control and survival rates, reducing long-term side effects becomes even more important for quality of life. The above-mentioned randomized trial by Hoskin et al. can already show similar toxicity rates for both EBRT alone and EBRT + BT boost. The prevalence of severe GU adverse events at 5 and 7 years was 9% and 4% for EBRT compared with 8% and 11% for EBRT + BT. No statistically significant differences were found, much as for the incidence of side effects. For comparison, in our cohort, the prevalence of GU toxicity grade ≥ 3 was 9% at 5 years. However, the retrospective nature of our analysis implies that, for example, data on pre-therapeutical potency in our elderly cohort was in general not available. It is important to differentiate treatment-related toxicity (limited to the early phase up to 3 months post-radiotherapy) from long-term toxicity. Usually, the prevalence of side effects after radiation therapy of the prostate gland is much lower after the acute phase. For example, Rodda et al. were able to show the difference of cumulative incidence of side effects and the prevalence of 2 and 5 years post-radiation therapy [14]. Compared with the cumulative incidence, the 5-year prevalence of grade 3 toxicity of GU tract and gastrointestinal (GI) tract was substantially lower in patients treated with EBRT and BT. In our cohort, the reported side effects prevalent at 5 years were 18% compared with the cumulative 5-year incidence of 50%. This proves how well patients recover after experiencing initial side effects. Especially the feared incontinence (cumulative 5-year incidence of 17% vs. 5-year prevalence of 6%) and impotence (cumulative 5-year incidence of 30% vs. 5-year prevalence of 6%) were not permanent for many patients in our analysis. In the past, brachytherapy for patients with a prostate volume of more than 50 cm3 was not recommended. Now, the current guidelines state that larger glands should also be considered for brachytherapy [27]. We can confirm these recommendations, since in our cohort, a large prostate volume was not correlated with more toxicity or worse survival outcomes.
In comparison with our results, the published rates of urinary incontinence after prostatectomy are 18-20% after 2-3 years [28, 29]. While after 2-6 years, only 15-19% chance of potency remains after prostatectomy, according to large study cohorts. In contrast, the cumulative incidence of impotence in our cohort was 17%. Compared with permanent low-dose-rate BT (LDR-BT), GU toxicity seems to be reduced after HDR-BT [30]. This supports our approach of combining EBRT and temporary BT. The combination of EBRT and BT allows total doses of 85 Gy and more with acceptable rates of side effects, which is in line with our data. Therefore, patients diagnosed with intermediate- or high-risk prostate cancer should be offered all information available on their treatment options, even though brachytherapy is offered in specialized centers [31, 32].
HDR-BT and LDR-BT are used for dose escalation more commonly than PDR-BT [27]. Published studies on using PDR-BT in prostate cancer are scarce, probably because HDR-BT data are easily available. Its biological effect has been well-studied [33, 34]. In a prospective trial with a median follow-up of 60 months, 130 patients were treated with PDR-BT as dose escalation after EBRT [20]. The 5-year bRFS rate was 85.6% and CSOS rate was 98.2%. These results are in line with those of the current study: the bRFS rate of 86.5% and CSOS rate of 97.3% at 5 years. Our data demonstrate that both PDR-BT and HDR-BT are equally safe and efficient.
The study’s cohort showed the typical real-life characteristics of old age and some inhomogeneity within treatment strategies, such as the use of hormonal ablative therapy. Therefore, survival data is not directly comparable with other prospective studies using strict inclusion criteria, especially regarding age and co-existing diseases. Nonetheless, our results, e.g., OS rates, are in line with other trials. Hoskin et al. [11] reported a 7-year OS of 88%, whereas we found 88.9% in our cohort. Due to retrospective nature of the analysis, information regarding toxicity was probably missed. In our experience, patients need to be asked clearly for specific side effects, such as potency issues. Furthermore, a baseline score was missing for most of the patients. In our clinic, the LENT-SOMA scale was used more frequently in the past, while currently, the RTOG/EORTC scale is utilized. This makes direct comparisons of our study results more difficult. Regarding the physician’s choice of treatment delivery type, we expect no bias, since availability in the department played the major role. Another limitation of the study is the inconsistent application of hormonal therapy. All patients were recommended using hormonal therapy according to the national guidelines, but many declined. Since this was a retrospective analysis, randomization was not possible.
With constant increase of life expectancy and growing rate of prostate cancer detection, the future prostate cancer patient will be more complex [35, 36]. As our data clearly shows, combination radiation therapy is feasible and well-tolerated in elderly patients also. All patients completed the prescribed boost radiation dose. The efficacy of our treatment is evident in the very low-rate of local recurrences.
According to current guidelines of the American Society of Clinical Oncology (ASCO), BT boost should be offered to all eligible patients with intermediate- or high-risk prostate cancer [37]. Even though BT is not available in every radiation department, discussing this method with patients is even more important, as our results show. We strongly support the perspective presented in the ASCO guidelines.
For the future, more precise diagnostics will change the treatment algorithms for prostate cancer. Specificity and sensitivity of the PSMA PET-CT and multi-parametric magnetic resonance imaging (MRI) exceed former imaging possibilities. Therefore, they should be integrated into pre-treatment staging in advanced prostate cancer as well as utilized for the definition of target volume. Sanmamed et al. used multi-parametric MRI scans to determine gross tumor volume (GTV) for boost irradiation applied either with EBRT or HDR-BT. The authors showed the advantage of HDR-BT compared with dose-escalated EBRT in treatment toxicity [38]. In the prospective HistoScanning trial, tumor lesions within the prostate gland were identified using computer-aided ultrasonography [39]. In the BT procedure, the included patients received a simultaneous focal boost with up to 119 Gy (EQD2α/β = 3). The authors reported efficacy and safety with a 5-year LRR of 1%, and late toxicity of more than grade 2 in only 4% of patients. More recent studies focus on the idea of applying even more radiation dose to specific tumor areas within the prostate gland [38-41], showing an improved bRFS without increased toxicities or decreased quality of life. In the largest study dedicated to focal dose escalation, the multi-center randomized FLAME trial, patients received an escalated dose up to 95 Gy to the visible tumor and 77 Gy to the whole gland [40]. The cumulative incidence of late genitourinary and GI toxicity grade ≥ 2 was 23% and 12% in the standard arm versus 28% and 13% in the focal boost arm, respectively [42]. These approaches might be the future of BT for the treatment of locally advanced prostate cancer.
Conclusions
In summary, the interstitial boost irradiation using brachytherapy is an effective and safe local treatment for localized prostate cancer, with minimal late side effects in elderly patients also. A prostate volume of more than 50 cm3 is no contraindication. Regarding efficacy and safety, PDR-BT and HDR-BT are equally beneficial. Therefore, brachytherapy boost should be consequently offered to all eligible patients.