Purpose
Neoadjuvant chemoradiation (NACTRT) followed by total mesenteric excision (TME) has long been the standard treatment for locally advanced rectal cancers. However, for patients with low-lying rectal cancers, this approach often necessitates the creation of a permanent stoma, associated with significant complications such as peristomal skin irritation, leakage, stoma necrosis, parastomal hernia, stomal prolapse, stomal stenosis and obstruction [1]. These complications contribute to significant physical and psychological distress, including depression, anxiety, and a reduction in quality of life (QoL) [2].
An alternative approach, the watch-and-wait (W&W) strategy or non-operative management (NOM), has gained traction recently, after Habr-Gama et al. showed that in patients who achieve a clinical complete response (cCR) after chemoradiation, W&W allows for the preservation of the anal sphincter, avoiding the need for a permanent stoma [3]. Brachytherapy can significantly improve the non-operative management (NOM) rates [4]. Various brachytherapy methods have evolved at various centres based on the availability of machines, logistics and expertise. For example, many UK centres use Papillon contact X-ray; in Canada, computed tomography (CT)-guided high-dose-rate (HDR) brachytherapy is used, and magnetic resonance imaging (MRI)-guided HDR brachytherapy is practised at our centre [5-9].
Our centre has been offering rectal brachytherapy for the past five years, with a remarkable increase in volume, from just 1-2 cases annually in the initial years to 60-80 cases per year currently. The initial phase (years 1-3) was largely exploratory, marked by discovery and evolution of processes. However, as a high-volume and logistically constrained centre, integrating rectal brachytherapy into an already stretched system posed significant challenges. The brachytherapy workstation is frequently overwhelmed with gynaecological and other site brachytherapy procedures, leaving limited availability for new and rarer procedures such as rectal brachytherapy. Additionally, the MRI station, which is an essential part of the workflow, is heavily booked with regular diagnostic appointments and must also accommodate night emergencies and anaesthesia procedures in morning slots, delaying planned office procedures such as rectal brachytherapy, which need a radiation oncologist to insert the applicator in MRI and validate the scan. We observed that at our centre, many patients could not complete the process of MRI acquisition, planning and treatment delivery on the same day, affecting patient and caregiver satisfaction. This could impact the overall acceptance of brachytherapy and the success of the NOM approach.
Classical quality-improving (QI) methodology, such as A3 methodology, has been previously demonstrated to be an effective tool to review the patient pathway processes systematically and arrive at care pathway intervention to improve the quality of care [10-13]. In this article, we describe the implementation of classic quality-improving (QI) methodology, which included defining the problem statement using the SMART goal (Specific, Measurable, Achievable, Relevant and Time-bound), root cause analysis using a fish-bone diagram and implementation of corrective measures based on Plan-Do-Study-Act (PDSA) cycles. These were aimed at optimising our MRI-based rectal brachytherapy process, thereby avoiding delays and improving the efficiency of the workflow process.
Material and methods
A multidisciplinary team was formed as part of a quality improvement initiative, which included radiation oncologists, radiologists, medical physicists, specialist technologists, and nurses. The data related to the radiotherapy planning MRI and treatment delivery dates/times were retrieved from real-time electronic time stamps as available (MRI information from the institution’s picture archival and communication system (PACS) and brachytherapy from the treatment console). This study was undertaken as a QI rather than human participant research; therefore, institutional review board approval was not sought.
All patients who received rectal brachytherapy from 1st August 2022 to 30th November 2022 were included for baseline data collection. Wait time for each patient was calculated by finding the number of days from radiotherapy planning MRI to brachytherapy delivery. Also, the number of patients who received brachytherapy on the same day as planning MRI was calculated and denoted as D0%. Calculations were done fortnightly and plotted as run charts to track temporal trends of Dmean and Dmedian of wait time and D0%.
The baseline data from 31 patients showed mean (Dmean) and median (Dmedian) wait times of 14 and 15 days, respectively. Also, no patient could receive same-day treatments (D0% = 0). The “SMART goal” identified was to reduce the Dmean and Dmedian of wait time to less than one day each, while the secondary goal was to increase the proportion of same-day treatments to at least 70% by 31st January 2023. The balancing measure was treatment errors or delays.
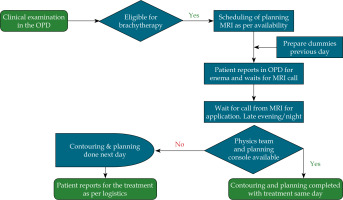
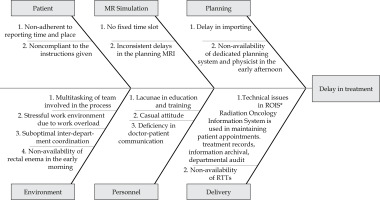
The core project team held weekly meetings to track progress. A detailed workflow schematic was developed for preparing patients for brachytherapy (Figure 1). A thorough analysis of the existing workflow was conducted to identify key bottlenecks and delays associated with MRI scheduling, treatment planning, and inter-departmental coordination. Factors contributing to delays were identified through gemba walks, summarising findings using a cause-and-effect (fishbone) diagram (see Figure 2). The core team identified the most frequent causes of delay (see Table 1) using a Pareto chart and designed interventions for those that were easy to implement and had the greatest benefit for the patients and care providers. A quality improvement strategy was implemented based on the Plan-Do-Study-Act (PDSA) cycle in response to these delays.
Table 1
The most frequent causes of delay based on the fishbone diagram were identified using a Pareto chart and listed as follows
As part of the PDSA cycle 1 interventions, key personnel were trained on the importance of timely MRI availability and the necessary steps to prepare for treatment efficiently, emphasising minimising downtime between imaging, planning, and treatment delivery. Efforts were made to optimise MRI availability for brachytherapy planning, including scheduling dedicated MRI slots in the early morning (8 a.m.-10 a.m.). The communication between the radiation and MRI departments was improved to ensure alignment on treatment timelines and responsibilities, minimising delays due to miscoordination. Patients were counselled on the importance of arriving early (6 a.m.-7 a.m.) in the day to allow enough time for preparation before the planning MRI. These steps were essential to ensure the feasibility of same-day treatments. All these interventions as a cluster were launched on 1st December 2022. PDSA cycle 1 lasted from 1st December 2022 to 31st December 2022. The primary measure was Dmean and Dmedian of wait time, and the secondary measure was D0%.
As part of PDSA cycle 2, all previous interventions from PDSA cycle 1 were reinforced, ensuring their continued implementation. A detailed SOP for brachytherapy was created and distributed to all team members to standardise practices and reduce delays. Better coordination with nursing staff was implemented to ensure the availability of rectal enema supplies during the early morning hours (6 a.m.-8 a.m.). The availability of the medical physicist in the forenoon (10 a.m.-12 noon) was ensured to allow for timely treatment planning, as in the afternoon, gynaecology implants would be ready for planning, and other cases could not be accommodated. PDSA cycle 2 lasted from 1st January 2023 to 31st January 2023.
Results
The first PDSA cycle included patient reminders to arrive at specific times, improved communication, and coordination with the MRI team for a fixed scheduled slot in the morning (8-10 a.m.). The second PDSA cycle included the availability of the rectum enema facility in the early morning (6-8 a.m.), strengthening coordination with the MRI team and re-enforcing the availability of a medical physicist and planning system in the early afternoon (10-12 noon). The post-implementation data at PDSA cycles 1 and 2 from 14 patients each were analysed. The post-change Dmedian, Dmean and D0% improved to 3 days, 3 days and 35.7%, respectively, for PDSA cycle 1. This further improved to zero, 0.2 days and 78.9%, respectively, for PDSA cycle 2.
The aim of this initial study was achieved. A sustained shift in the process was apparent on a control run chart (Figure 3: Dmean and Dmedian; Figure 4: D0%) for 8 months (Sept 2023), suggesting sustainability. The balancing measure remained the same. Further in the sustenance phase, the Dmedian, Dmean and D0% were maintained at 0.3, 0 days and 74%, respectively, for the next 42 patients.
Fig. 3
Run chart showing median and mean of the duration between planning MRI and brachytherapy treatment
The x-axis shows the timeline (dd/mm/yyyy) and the y-axis shows the number of days. The Dmedian and Dmean at baseline were 14 and 15 days, respectively, which improved to 3 and 3 days after PDSA cycle 1 and to zero and 0.2 days after PDSA cycle 2.
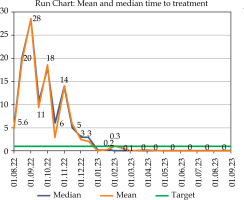
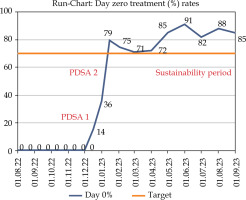
Fig. 4
Run chart showing the number of same day treatment rates (denoted as D0%)
The x-axis shows the timeline (dd/mm/yyyy) and the y-axis shows the percentage. The same day treatment rate at baseline was 0%, which improved to 35.7% and 78.9% after PDSA cycle 1 and PDSA cycle 2, respectively, after which it was sustained over 70%, as targeted.
Discussion
MRI-based brachytherapy for rectal cancer is a new procedure requiring inter- and intra-departmental coordination and cooperation. Increased wait time leads to dissatisfaction among patients and the treating team, potentially leading to a decrease in the popularity of the procedure and may impact the acceptance and success of the W&W approach. Using the classical quality improvement methodology (Figure 5), we successfully reduced the delay between the planning MRI day and the treatment delivery day in a sustainable way. Following the implementation of PDSA cycle 2, 74% of patients received same-day treatment. Subsequent internal audits demonstrated sustained improvement, with over 95% of patients consistently receiving treatment on the same day as the planning MRI.
Fig. 5
A3 figure for timely implementation of MRI-based rectal brachytherapy in rectal cancer patient for W&W approach

The most frequent factors leading to increasing wait times were inconsistency in MRI scheduling, poor interdepartmental coordination, non-standard patient arrival times, lack of facility availability for enemas in the early morning hours and limited availability of medical physicists in the early afternoon.
Implementing a quality improvement methodology has successfully addressed our institution’s logistical barriers associated with MRI-based rectal brachytherapy. By reducing delays, we have enhanced the treatment experience for patients and improved workflow efficiency. The success of this initiative underscores the importance of continuous process optimisation in ensuring the timely delivery of effective treatments, particularly in complex cancer care regimens.
Rectal brachytherapy processes can be significantly enhanced using quality improvement (QI) methodology by systematically identifying inefficiencies in workflow. Tools such as process mapping help visualize every step from patient preparation to treatment delivery, allowing identification of factors causing delay in initiating treatment. Interventions such as checklists, standardized operating procedures (SOPs), and real-time audits ensure consistent performance and reduce the likelihood of delay. Multidisciplinary collaboration is central to QI, as it promotes shared ownership and improves communication among the personnel involved. By applying iterative PDSA cycles, institutions can optimize scheduling, resource allocation, and patient throughput, thereby avoiding delay. Ultimately, QI methodology not only streamlines operational efficiency but also enhances patient satisfaction, treatment quality, and staff accountability across the brachytherapy programme.
A few unplanned advantages emerged over the course of this exercise. While these were not formally analysed, they are noteworthy. For example, our initiative contributed to reducing disruptions in the gynaecological brachytherapy workflow caused by rectal cases, a particularly valuable outcome in a high-volume centre like ours. Although a formal cost analysis was not undertaken, we anticipate a tangible decrease in out-of-pocket expenses, especially for outstation patients, no longer needing to extend their stay due to treatment delay. Arguably, the most significant benefit of this initiative has been the avoidance of unnecessary delays across the treatment process, which in turn has supported greater acceptance of the W&W approach for both patients and the care team.
To summarise, the W&W approach remains a promising alternative to TME for patients with low-lying rectal cancers [3], and optimising the brachytherapy process is critical to its widespread adoption. Our findings suggest that quality improvement strategies can substantially reduce delays, improve patient satisfaction, and ultimately enhance clinical outcomes. This article may be useful for other centres looking at developing similar MRI-based brachytherapy in their departments for the W&W approach in suitable rectal cancer patients.
Conclusions
Optimisation of the MRI-based rectal brachytherapy process through quality improvement methodologies resulted in reduced delays in treatment planning and delivery. These improvements may serve as a model for other institutions aiming to implement, or having already implemented, MRI-based brachytherapy programmes for the W&W approach in suitable rectal cancer patients.
Data sharing statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Previous presentation: ESTRO 2024 poster.