Purpose
Endometrial carcinoma (EC) is the fourth most common cancer among Italian women, with an increasing incidence over the last 10 years [1]. Surgery is currently the standard treatment, and consists of hysterectomy and bilateral salpingo-oophorectomy (TH/BSO) with or without pelvic and/or para-aortic lymphadenectomy, and peritoneal washing [2, 3]. Majority of Italian patients are diagnosed with an early stage disease, with a reported 5-year overall survival of 77%, similar to that reported in other Western countries [4]. Results of post-operative radiation therapy in endometrial carcinoma (PORTEC) study group trials, gynecologic oncology group (GOG) trial, and a Swedish study have shown that adjuvant vaginal brachytherapy (VBT) alone provides equivalent local disease control compared to external beam radiation therapy (EBRT) or to combined treatment, with no significant difference in overall survival between arms [5-8]. Moreover, when compared with EBRT, VBT was associated with a lower rate of acute gastrointestinal, genitourinary, and hematological toxicities [8-10]. Therefore, exclusive VIRt is currently considered the standard adjuvant treatment in intermediate- or high-intermediate risk patients [2, 3]. VBT has been related to local control ranging from 90% to 100%, with low-rate of late vaginal toxicity, mainly G1-G2 (range, 7.5-27.7%), and very low rates (range, 0.5-2%) of severe late toxicity, above all vaginal stenosis and atrophy [11, 12]. Although the most commonly employed fractionation is considered 21 Gy as total dose with 7 Gy per fraction delivered once a week. However recently, different fractionations characterized by a reduction of total treatment time, as three times a week, were offered in clinical practice without detrimental impact both on local control and survival. Above all, no significant increase of late toxicities were reported [5-8, 13-17].
Also, in our consecutive series of 55 patients with early stage intermediate- and high-intermediate risk EC treated with exclusive HDR-VBT to a total dose of 21 Gy, 7 Gy per fraction every other day, we reported satisfying local control, survival outcomes, and toxicities [17]. Although survival and acute and late toxicity profiles are the primary endpoints of EC treatment, the evaluation of effects of therapy on patient’s health-related quality of life (HR-QOL) and sexual functioning has become increasingly important, especially in patients with early-stage disease with longer expected survival [18-20]. Few studies have assessed quality of life (QOL) and sexual dysfunctions in patients treated with exclusive VBT in early stage EC [21-25]. The primary endpoint of our study was to record eventual changes in QOL and sexual functioning at different time points in a consecutive series of patients with International Federation of Gynecology and Obstetrics (FIGO) stage I intermediate- or high-intermediate risk endometrial cancer, treated with 21 Gy one week high-dose-rate (HDR) VBT. Secondary endpoints were overall survival (OS), metastasis-free survival (MFS), and loco-regional control.
Material and methods
Patients
Between July 2008 and October 2013, 55 patients with diagnosis of endometrial cancer were treated with adjuvant exclusive VBT at our institution. All patients had undergone surgical treatment with a laparotomy approach before VBT. Pathological stage was defined according to FIGO 2009 surgical staging system.
Overall, stage distribution was as follows: IA in 27 (49%) patients, and IB in 28 (51%) patients. All patients presented endometrioid pathological sub-type. Post-operative VBT was administered in all patients at 6-8 weeks after surgery. Three-dimensional planning was performed after acquisition of CT-based imaging, with 0.25 cm slices for all patients. VBT was delivered to vaginal vault using a Nucletron HDR unit, with iridium-192 source at a dose of 21 Gy given in three fractions of 7 Gy each, three times a week, every other day, prescribed at 0.5 cm depth of vaginal wall and 3 cm in length from the apex [26]. The protocol was approved by a local ethics committees. Every patient signed a written informed consent.
First follow-up evaluation was done at 6 weeks after completion of VBT treatment. Thereafter, all patients were evaluated every 4 months for the first 2 years, and every 6 months afterwards for at least 5 years, with clinical and pelvic examination and vaginal cytology. Chest radiography and pelvic and abdominal ultrasounds were carried out annually, with additional CT and/or MRI whenever required.
Quality of life assessment
Health-related quality of life was assessed using European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire Core-30 (QLQ-C30, version 3), and EORTC cancer-specific quality of life questionnaire (QLQ-CX24), the latter specifically dedicated to gynecologic patients [27, 28]. In particular, the QLQ-C30 consists of 5 functional scales (physical, role, emotional, cognitive, and social), 3 symptom scales (fatigue, nausea and vomiting, and pain), 6 single-item symptom scales (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial effect of treatment), and global health status. Moreover, as EC-specific EN24 module was not yet available, the EORTC QLQ-CX24 was applied, including 18 items grouped into three domains, symptom experience (abdominal/gastrointestinal symptoms, peripheral neuropathy, other chemotherapy side-effects, hormonal/menopausal symptoms, and hair loss), body image (body image, sexuality, and attitudes concerning the disease and its’ treatment), and sexual/vaginal functioning (vaginal dryness, vaginal shortness, vaginal tightness, and vaginal pain) as well as six single-item scales (lymphedema, peripheral neuropathy, menopausal symptoms, sexual worry, sexual activity, and sexual enjoyment). We selected the following domains that patients with endometrial cancer undergoing VBT are likely to be the most affected with: global health status, physical functioning, role functioning, emotional functioning, social functioning (as functional scales), constipation and diarrhea (as single-item symptoms for QLQ-C30); symptoms’ experience, body image, and sexual/vaginal functioning (as main domains), and sexual worry, sexual activity, and sexual enjoyment (as single-item scales for EORTC QLQ-CX24).
Patients were prospectively requested to complete the questionnaires immediately before VBT, 4 weeks after VIRt, and thereafter during post-treatment follow-up every 3 months in the first year, and every 6 months, up to 60 months follow-up. QLQs were scored according to the recommended EORTC procedures [29]. All raw scores were transformed to scores ranging from 0 to 100. For functioning scales, global QOL scale, and for items on sexual activity and sexual enjoyment of QLQ-CX24, a higher score represented a better level of functioning and a better QOL, whereas for symptom scales/items, a higher score indicated a worse level of symptomatology.
Statistics analysis
Continuous data were summarized as means and standard deviation. Categorical data were depicted using absolute frequencies and relative percentages. Statistical comparisons for quantitative data were performed using Wilcoxon rank sum test. Analysis of QLQ-C30 and QLQ-CX24 were done according to the EORTC quality of life group guidelines. Longitudinal analysis of the scores were performed using a linear mixed model with patients as random effect and time (in months), age (qualitative, age > 70), body mass index (BMI) (qualitative, BMI > 30), and their interaction as fixed effects. All statistical analyses were performed with R v. 4.0.1 and package lme4, and p-values lower than 0.05 were considered significant. Survival analysis for outcomes was performed considering overall survival (OS), calculated from the date of histological exam to the date of death from any cause or the date of last follow-up. Metastasis-free survival (MFS) was calculated from the date of the end of brachytherapy course to the date of either distant metastases or the date of the last follow-up. Kaplan-Meier product limit estimates were applied to estimate the rates of OS and DFS. SPSS statistical software package version 13.0 (SPSS, Chicago, IL, USA) was used for survival analysis.
Results
In total, 55 patients were enrolled into the present study. Patient and tumor characteristics are shown in Table 1. The median age at diagnosis was 66 years (range, 35-79 years). Twenty-seven (49%) patients presented FIGO stage IA, and 28 (51%) patients IB.
Table 1
Patient and tumor characteristics
The questionnaires were carried out respectively at 1, 3, 6, 12, 24, 36, 48, and 60 months after the end of brachytherapy. The response rate to the questionnaire was 100% (n = 55). Nineteen patients (35%) answered all the questions of surveys, while 36 patients (65%) completed the surveys except for questions on sex activity, vaginal function, and sex enjoyment.
Longitudinal analysis during the 5-year follow-up period showed a statistically significant trend towards worsening of fatigue, constipation, and diarrhea (Table 2). The worsening of fatigue was not significant in obese patients, while the increased constipation was strongly enhanced in patients older than 70 years. Overall physical functioning and role functioning were not impaired after VBT. A worsening of physical functioning and role functioning were observed only in the sub-group of obese (BMI > 30) patients. Over the time, sex enjoyment improved, except for elderly patients. For emotional functioning, sex worry, and social functioning, there were no significant time-related effects.
Table 2
Longitudinal analysis
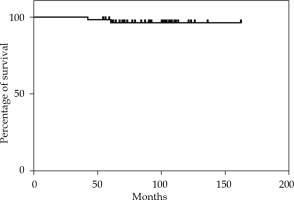
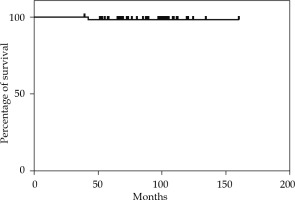
The median follow-up time was 92 months (range, 42-162 months). The 2-year, 5-year, and 10-year OS rates were 100%, 98.18%, and 96% (Figure 1). The 2-year and 5-year MFS rates were 100% and 98.14% (Figure 2). None of the 55 patients presented a loco-regional relapse. Two patients died, one from causes not related to the disease (cardiological disease) and the other from a systemic disease.
Discussion
In the present study, we have prospectively assessed HR-QOL, sexual functioning, and fatigue in 55 patients with stage I endometrial cancer treated with adjuvant exclusive HDR-VBT.
Surgery is significantly related to worsening of QOL, mainly of physical and functional domains, particularly during the first 6 months [16]. In our series, physical functioning, role functioning, and social functioning scores were not impaired in almost all patients after surgery, meaning that the patients showed a fast recovery after surgery from their previous habits. Moreover, VBT had a little influence on their habits in social life and work overtime. In total, emotional functioning was not impaired, except for obese patients who reported significantly worsened emotional functioning after VBT, with no recovery overtime.
Sexual dysfunction in gynecologic cancer is thought to result mainly from adjuvant treatment, such as surgery and/or radiation therapy, although the real impact of these adjuvant treatments on sexual disfunction is not well-defined till now [11-28]. In a series of 101 endometrial patients with stage I-IIIA, sexual dysfunction was recorded in 89% [23]. All the patients underwent surgery, but only 18% of them received adjuvant therapy (pelvic radiotherapy and/or VBT). Similarly, Becker et al. [2] and Quick et al. [25] in their series of early stage endometrial cancer patients treated with surgery alone or surgery plus BT, underlined that the actual impact of BT on sexual function was negligible. On the contrary, Damast et al. [7] showed 81% of sexual dysfunction (mainly vaginal dryness and dyspareunia) in 101 patients with stage I-II EC treated with surgery and VBT. The rates of sexual activity and symptom scores reported by PORTEC-2 trial patients were similar to the long-term scores of PORTEC-1 trial survivors, also those treated with surgery alone, suggesting an impact of cancer diagnosis and treatment on sexual aspects of HRQL.9.
In 2018, Delishaj et al. published a comprehensive literature review including 13 studies and about 2,400 patients treated with adjuvant vaginal BT, and analyzed BT-related vaginal toxicities. The most commonly used BT course consisted of 21-22 Gy, delivered in three fractions, two/three times per week (range, 10-34 Gy in 2-6 fractions, two-six times per week). Even though using different courses of BT was safe and well-tolerated, grade 1-2 acute vaginal toxicities varied widely from 8.7% to 20.6%, and most common were vaginal inflammation, vaginal irritation, dryness, discharge, soreness, swelling, and fungal infection. Late toxicities reported consisted of vaginal discharge, dryness, itching, bleeding, fibrosis, telangiectasias, stenosis, short or narrow vagina, and dyspareunia, and ranged from 7.5% to 27.7%. Notably, the rates of late toxicities between the studies were related to broad different biologically effective prescribed doses (BED), ranging from 15 Gy to 92 Gy [29].
A recent study of Lancellotta et al. compared late vaginal toxicities rates after two different HDR-BT dose schedules (21 Gy delivered in three weekly fractions of 7 Gy vs. 24 Gy delivered in four weekly fractions of 6 Gy). Vaginal late toxicities occurred in both the groups in first years of follow-up, but when comparing the two schedules, significant differences in late vaginal toxicity rates (53.3% vs. 23.6%, respectively) were observed, favoring 24 Gy in 4 fractions vs. 21 Gy in 3 fractions [30].
Papathemelis et al. analyzed QOL questionnaires of 104 patients with EC after brachytherapy, with two different high-dose-rate schemes (15 Gy/3 fractions once a week vs. 20 Gy/4 fractions once a week). Similarly with our series, no difference in QOL was observed between the two schemes. Only younger patients (< 70 years) had a significantly lower emotional functioning scale and experienced higher symptom scales of diarrhea [31].
In regard to sexual activity and sexual satisfaction, in our study, these items did not worsen significantly after BT, and on the contrary, there was a trend towards improvement of sex enjoyment, even though limitations to any conclusion are a low-rate of sexual activity in elderly population, and a lower completion rate of sexual functioning questions. Moreover, the results of our study showed that there was no statistically significant impact on vaginal irradiation on vaginal function, body image, and symptom experience domains.
The present study has some limitations that need to be taken into account when interpreting the results. First, the study did not include a control group. Moreover, baseline assessment of QOL condition in our series of patients was made after surgery, so we cannot verify the actual impact of surgery.