Purpose
A radical cystectomy (RC) with pelvic lymph node dissection, recently increasingly preceded with neo-adjuvant chemotherapy, is the golden standard for patients with a muscle-invasive bladder carcinoma (MIBC) [1]. In the past decades, several bladder-sparing treatments have been developed, including the combination of external beam radiation therapy (EBRT) and brachytherapy (BT), a modality undergoing continuous improvement since the beginning of this century [2]. Here, we reported the outcomes of a quality of life (QoL) study performed among patients treated with bladder-sparing treatment.
Patients with a solitary T1G3 and T2 carcinoma smaller than five centimeters are eligible for a BT-based bladder-sparing treatment [3, 4]. This treatment consists of trans-urethral resection of the bladder tumor (TURBT), always followed by EBRT (20 × 2 Gy) and a high-dose-rate (HDR) BT boost: 7 × 2.5 Gy in case of partial cystectomy (PC), otherwise 10 × 2.5 Gy [3, 5]. Brachytherapy procedure is based on an already previously meticulously described robot-assisted laparoscopic technique [2, 5]. Several publications have already demonstrated the oncological effectiveness of this treatment [6-10], reporting an average 5-year disease-specific survival of 75.2%, a local failure rate of 14% [11], and less toxicity and complications compared with RC [6, 10]. However, this treatment option is not always discussed with patients, and is thereby confined to highly specialized centers, predominantly in The Netherlands [11].
Quality of life is an increasingly important concern in the choice of treatment. The QoL of bladder cancer patients who have undergone RC has been studied several times, particularly comparing neo-bladder and ileal conduit QoL [12-14]. However there is no research on the population who received BT-based bladder-sparing treatment. According to our clinical perception, this group of patients is expected to have a good QoL.
The primary aim of the present study was to obtain QoL data of our treated population. The secondary aim was to compare the outcomes with QoL data of a representative age-matched cohort from a general Dutch population.
Material and methods
Research design and research population
This research was a single-center, prospective, descriptive, cross-sectional study. A total of 98 patients with cT2 (n = 97) and recurrent cT1G3 bladder tumor (n = 1) smaller than five centimeters were treated with BT-based bladder-sparing therapy between January 2016 and June 2021. Of these, 83 patients who were still alive were approached to complete a QoL questionnaire, excluding those who underwent salvage cystectomy. In total, 82 patients received a letter, except one patient due to incorrect address.
All patients were treated with TURBT, followed by EBRT and HDR BT boost. EBRT consisted of a total dose of 40 Gray in 20 fractions over four weeks, using intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc therapy (VMAT) technique, with daily position verification by cone beam computer tomography. HDR schedule was either 10 or 7 fractions of 2.5 Gy, 3 fractions per day on consecutive days, and the latter after PC. An iridium-192 (192Ir) HDR flexisource was applied for the BT treatment [15-17]. The source activity was between 1.1 Curie and 0.3 Curie.
Data collection
All patients who were eligible to participate were approached by post. They received an information letter and questionnaires. The information letter clearly stated that participation was voluntary, and data would be processed anonymously. In this study, three questionnaires were combined to measure general and MIBC-specific QoL. In addition, demographic data was collected. The procedure was reviewed by medical research ethics committee of our hospital.
Quality of life questionnaires
For this research, three questionnaires were used. General QoL was measured with the European Organization for Research and Treatment of Cancer QoL questionnaire (EORTC QLQ-C30) [18]. MIBC-specific QoL was assessed with the EORTC QLQ-BLM30 [19], and bowel problems were evaluated with the bowel domain from the expanded prostate cancer index composite (EPIC-50) questionnaire [20].
The EORTC QLQ-C30 consists of five function scales, including three symptoms’ scales, six single-item measures, and a global health status/QoL score. The EORTC QLQ-BLM30 consists of seven-symptom scales, of which the symptom scales, such as ‘urostomy problems’ and ‘catheterization’ were not considered in this study, because patient’s bladder has not been removed. The sub-scale ‘sexuality’ is represented by six single items. The bowel domain of the EPIC-50 questionnaire yields three scores. All questions are closed questions, and are scored based on a Likert’s scale. The raw scores per sub-category were converted to a score between 0-100. The EORTC-QLQC30 and the EPIC-50 are validated questionnaires. The EORTC QLQ-BLM30 has not yet been validated, but testing of phase three is completed.
Statistical analysis
Baseline characteristics of the study population were determined using descriptive statistics, including frequencies, median, and range. Global health status/QoL score was calculated according to the EORTC instructions for question 29 (global QoL) and question 30 (global health) of the generic EORTC QLQ-C30 v.3 questionnaire [21]. For the assessment of global health status/QoL score, the definition of Snyder et al. [22] was followed. In line with this definition, a score of ≥ 70 was associated with a high QoL, and a score of < 70 was associated with a low QoL. Therefore, a high score of the function scales and a low score of the symptom scales were associated with a high QoL [23].
Subsequently, means and 95% confidence interval (CI) were determined for variables of the questionnaires. In addition, the variables were presented in function and symptom scales as described in the EORTC manual, with a score ranging from 0 and 100. When 50% or more items of a multi-item scale were not answered, the value of that respondent was shown as missing value.
The calculation of scores for symptom scales of the EORTC QLQ-BLM30 was done according to the same conditions as the scores of EORTC QLQ-C30. In the EPIC-50, a score was shown as a missing value when ≥ 20% of the items of relevant (sub)score were not answered [24].
The mean scores of general Dutch population [25] were adjusted to age distribution of BT patients. This was done by calculating the expected mean score as described by Schou et al. [26]. One-sample t-test was used to compare the mean function, symptoms, and two sexual scores of BT patients with the expected mean scores of general Dutch population. In addition, clinically relevant differences were examined. For the function and symptom scales, a difference of ten points was considered clinically relevant [27].
To investigate the impact of a partial cystectomy on the global health status/QoL score, the study population was divided into two groups. Differences in the mean global health status/QoL scores of both sub-groups were analyzed using Mann-Whitney U test. This was also done for variables, such as gender, age, body mass index (BMI), time since BT treatment, and sexual activity.
Finally, the relationship of global health status/QoL score with other variables was determined using Spearman’s rank correlation coefficient (r). All reported p-values were two-sided, with a significance level of 5%. Statistical analyses were performed with SPSS version 24.
Results
Baseline characteristics
Sixty-three (76%) of 82 approached patients responded to the questionnaires; three of them underwent a salvage cystectomy and were excluded from data-analysis. The median follow-up time for all analyzed patients was 32 months (range, 0.75-62 months). Table 1 shows the baseline characteristics of the study population. Non-responders were younger with a shorter median follow-up time compared with patients who participated in the study.
Table 1
Baseline characteristics of the study population
Quality of life outcomes
Table 2 demonstrates the mean scores and corresponding 95% CI for the function and symptoms scales. The highest levels of functioning scale appeared in the emotional, cognitive, and social domains. Fatigue was reported by 60% of the participants, and was therefore the most frequently occurring symptom of the symptoms measured for general QoL. Symptoms, such as nausea and vomiting, loss of appetite, and financial difficulties were not experienced by most of the participants.
Table 2
Mean scores and 95% CI of QLQ-C30, QLQ-BLM30, and EPIC-50 bowel domain of the bladder brachytherapy patients
The most common bladder cancer-specific symptoms occurring in more than 90% of the participants were urinary symptoms, of which 3.7% had a score above 60.0. Sixty-six percent of the participants indicated that they had no problems with body image. Twenty-three of 41 participants who completed the questions about sexuality were sexually active (19 men and 4 women). The gender-specific complaints for men are shown in Table 2. The female-specific complaint was reported by three women, whose data are not shown in Table 2. These women experienced some degree of vaginal dryness during sexual intercourse.
Quality of life outcomes vs. general Dutch population
Data from a study by Van de Poll-Franse et al. [25] was used to compare QoL of the participants with general Dutch population. Table 3 indicates the average scores of both the groups and related p-values. Significant differences were indicated with an indication. None of the mean scores differed by more than nine points.
Table 3
Mean scores of age-matched Dutch population [22] vs. bladder brachytherapy patients
Differences in global health status/QoL between groups within the study population
Within the research population, the participants were divided into different groups; the group was re-classified each time, and evaluated whether the global health status/QoL score differed between the two groups. This was done for gender, age, BMI, interval after BT treatment, partial cystectomy or fully bladder preservation, and sexual activity. Table 4 shows the median, interquartile range, and associated p-value. There were no significant differences between the study groups.
Table 4
Differences in global health status/quality of life (QoL) score between groups within the study population
Factors related to the EORTC QLQ-C30 global health status/QoL score
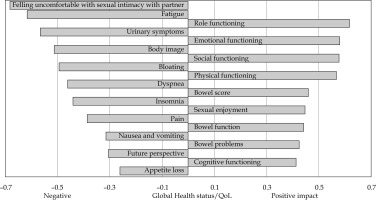
Figure 1 demonstrates all domains that had a significant correlation with the global health status/QoL score. In total, eleven domains were negatively correlated with the global health status/QoL. Feeling uncomfortable with sexual intimacy with partner (r = 0.683) had the strongest negative correlation, and loss of appetite presented the weakest negative correlation (r = –0.261). In total, nine domains were positively correlated with the global health status/QoL. The strongest positive correlation was with role functioning (r = 0.617), and the weakest positive correlation was with bowel problems (r = 0.426).
Discussion
This study was performed to assess information about QoL of MIBC population treated in our institute with BT-based organ sparing procedure. We used a combination of widely used and validated questionnaires. With a mean global health status/QoL score of 80.6, according to the definition of Snyder et al. [22], our patients presented good QoL. Notably, high scores were reported on the functional scales, including physical, role, emotional, and cognitive and social functioning, while the main reported complains were related to fatigue and urinary symptoms.
Compared with the general Dutch population, several significant differences are visible. A possible explanation for the significant differences in favor of BT patients may be that confrontation with the disease and treatment have changed the experience of wellbeing. Differences of ten points or more are considered clinically relevant., and in no case, the mean scores differed by more than ten points. Therefore, it can be assumed that QoL is not clinically different from the average Dutch population. However, given the limited number of MIBC patients eligible for this treatment, unavoidable significant differences in the sample size of the two populations must be taken into account.
This research shows that multiple factors are associated with the global health status/QoL score, both negative and positive. Within our study, only univariate analyses were performed to determine variables that have a correlation with the global health status/QoL. It is important that multivariate analyses are also performed to identify the variables that best predict the probability of a good QoL.
In comparison with RC patients, the difference in mean score for male sexual problems is particularly striking. In a study by Mak et al. [28], mostly involving patients with T2 tumors, an average score of 91 was found for erection problems and 69 for ejaculation problems. A study by Catto et al. [29] with mainly T1, T2, or unknown tumor stages, showed an average score of 77.6 for male sexual problems. Our patient group had a mean score of 40.0 for erection difficulties and 37.8 for ejaculation problems. Due to the magnitude of the differences, we hypothesize that these differences are likely to be clinically relevant. The mean scores for the role and social functioning also differ for BT-based treatment compared with RC. Catto et al. [29] found mean scores that were at least ten points lower, and the differences from Mak et al. [28] was respectively ten and five points in favor of our population.
The highest symptom scores reported by our patients included urinary and fatigue complaints. Both scores have a high negative correlation with the global health status/QoL score. For both types of complaint (urinary symptoms for RC patients who received a neo-bladder), Catto et al. [29] and Mak et al. [28] found scores within a ten-point difference, and thus may not be clinically relevant.
Mak et al. [28] conducted research on QoL after trimodality treatment (TMT), i.e., TURBT followed by chemotherapy and radiotherapy, which is currently an emerging organ-sparing treatment for MIBC. The mean scores only showed a difference of more than ten points for erection problems. They reported a mean score of 60, whereas we found a score of 40. This difference may be clinically relevant, but needs to be investigated in a comparative study. A study by Mak et al. [28] did not investigate complaints, such as dyspnea, insomnia, concerns about future perspective, body image, and sexual enjoyment. A comparative study between TMT, RC, and BT should be performed in order to directly compare QoL between these different modalities. Future studies comparing these three modalities might provide more extended QoL data.
An important limitation of this study is its cross-sectional design, which means that no solid baseline QoL data is available for BT patients. Therefore, the findings are hypothesis-generating, and would hopefully be the onset of prospective studies to investigate both baseline and long-term QoL outcomes for MIBC patients.
In addition, there were large differences in the time between the treatment and the completion of questionnaires (median follow-up of 32 months). Therefore, we compared the global health status/QoL score of the study population with a follow-up time of < 36 months and ≥ 36 months. However, there was no significant difference between the groups, a longitudinal study should clarify the differences in QoL scores at different follow-up times.
For further research, we plan a prospective longitudinal study. QoL must be determined at baseline and at several predefined follow-up times. Change of scores in time can also be included in the description of QoL of this patient group. In addition, it is desirable to establish a study that compares RC patients with organ preserving therapies.
Conclusions
To the best of our knowledge, this is the first and only study investigating QoL in patients with MIBC treated with a BT-based bladder-sparing procedure. With the mean global health status/QoL score of 80.6, the BT patients have a good QoL. We found no clinically relevant differences between the BT patient group and the age matched general Dutch population. This outcome strengthens the idea that this treatment option should be discussed with all patients eligible for BT-based bladder-preserving treatment.