Purpose
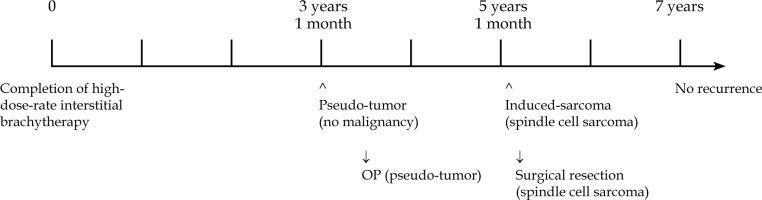
Among oral cancers, tongue cancer is most prevalent, with histopathological findings showing squamous cell carcinoma (SCC) to be responsible for 90% of these cancers. While surgical resection is considered to be the standard treatment for tongue cancer [1], radiation therapy may be utilized in some cases because of its’ advantages regarding the preservation of function and morphology. Previous studies have noted three-year local control rates following interstitial brachytherapy (ISBT) for tongue cancer of 82-84% [2, 3]. Both low-dose-rate ISBT (LDR-ISBT) and high-dose-rate ISBT (HDR-ISBT) are available. The advantages of LDR-ISBT include its’ simplicity, low level of anesthesia required, and low cost of the apparatus. Among its’ disadvantages are the operator cannot avoid being exposed to radiation, a shielded room is required for long-term patient irradiation, and dose distribution cannot be changed. HDR-ISBT overcomes these disadvantages, with irradiation time of only a few minutes, and it has recently come to be considered one of the most effective treatments for oral cancer [3]. It is important to note that radiation therapy has some side effects. Those occurring early during irradiation for oral cancer treatment include stomatitis and taste disorders, while late side effects include soft-tissue ulcers and osteonecrosis [4]. However, radiation-induced malignancy is probably the most serious complication affecting long-term survivors [5]. There is no consensus definition of radiation-induced malignancy, but the most important point is to distinguish it from recurrence. Therefore, the minimum requirement for diagnosis is a long period between the initial treatment and the occurrence of a new malignancy, and recently presented criteria include a latency period of several years [6, 7]. Furthermore, if a histopathological finding of a new malignancy is different from previous initial cancer, it is considered to be a radiation-induced malignancy [8]. Several reports of radiation-induced malignancy due to external beam radiation have been presented [9], while there are few reported cases following brachytherapy [10]. A search of the literature revealed no previous report of radiation-induced malignancy following HDR-ISBT for tongue cancer. The purpose of this study was to document information of a patient with tongue SCC, who was successfully treated with radiation therapy, but developed a radiation-induced undifferentiated spindle cell sarcoma of the tongue five years and one month later. Furthermore, a pseudo-tumor developed between the time of radiation exposure and the development of undifferentiated spindle cell sarcoma.
Case report
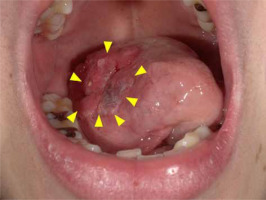
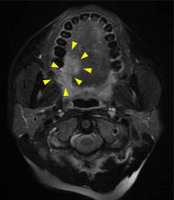
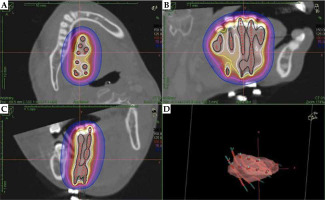
A 39-year-old woman visited our hospital with pain on the right side of her tongue. A clinical photograph showing a reddish irregular mass lesion in the right lateral border of tongue is presented in Figure 1, while T2-weighted magnetic resonance imaging (MRI) results showing a tumor sized 24 × 14 × 22 mm at the right lateral border of tongue (yellow arrows) are presented in Figure 2. Based on the biopsy and imaging results, the patient was diagnosed with SCC (cT3N0M0, stage III). A computed tomography (CT) scan was acquired, and a three-dimensional treatment plan was performed using Oncentra Brachy (Elekta AB, Stockholm, Sweden). The contour of clinical target volume (CTV) was identified using CT images. Planning target volume (PTV) was defined as an equivalent to CTV. We delivered 6 Gy as D100, i.e., minimum dose of the prescribed dose that was given to 100% of PTV. The patient underwent HDR-ISBT (60 Gy, 10 fractions, 10 flexible applicator tubes, double-plane) for 8 days, performed on the right side of the tongue (Figure 3).
Fig. 3
Dose-distribution diagram for high-dose-rate interstitial brachytherapy. Axial (A), sagittal (B), and coronal (C) sections showing dose distribution profile. A three-dimensional rendering image showing position of 10 flexible applicator tubes in relation to planning target volume (D)
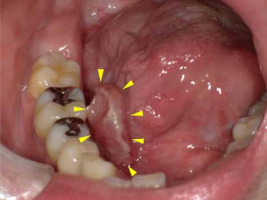
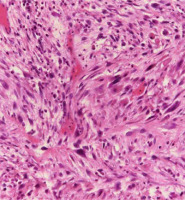
During follow-up, the patient noticed a mass lesion in the right side of the floor of the mouth three years and one month after the completion of therapy (Figure 4). An elevated lesion on the right side of the floor of the mouth was resected five months later, and based on surgical specimen, it was histopathologically diagnosed as high-grade dysplasia of the mucosa with suspicion of reactive hyperplasia. The tumor consisted of spindle-shaped atypical cells without malignancy (Figure 5). It was thought that the tumor was a reactive granulation containing atypical stromal cells caused by radiation, since the patient had a history of radiation. The lesion was small and superficial, with no apparent invasion into deeper muscle layers, and the shape was outwardly protruding.
Fig. 5
Histologic image. The mass shows spindle-shaped atypical cells with enlarged and densely stained nucleus proliferation, accompanied by neutrophil-dominated inflammatory cell infiltration (HE stain, 200×)
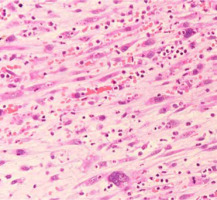
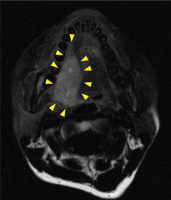
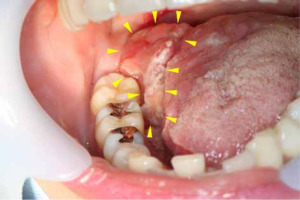
After five years and one month of follow-up after radiation therapy, re-growth of a lesion with induration on the right lateral border of tongue was observed (Figures 6 and 7). A total glossectomy, rectus abdominis muscle flap reconstruction, partial mandibulectomy, scapular reconstruction, and bilateral radical neck dissection were performed. The histopathological diagnosis was an undifferentiated spindle cell sarcoma (T4aN0M0) (Figure 8). One year and ten months after the surgery, there was no obvious recurrence or metastasis (Figure 9).
Fig. 7
T2-weighted magnetic resonance image obtained prior to second operation. The image results show lesion at the right lateral border of tongue measuring approximately 36 × 18 mm in diameter (yellow arrow). Comparisons with pre-operative T2-weighted images show that the location of the current tumor was nearly the same as that of the primary tumor
Discussion
According to criteria for radiation-induced malignancy presented by Sakai et al. [8], such induced cancer can be classified into three groups (A, B, and C) based on the histopathology, organ of origin, latency period, and irradiation field (Table 1). This classification is based on a definition of multiple primary malignancies presented by Warren and Gates [11]. This definition is considered to be very reliable, as it includes histopathological findings. The present case was classified as having an A2 reliability level, with a latency period of five years, which is consistent with the definition put forward by Sakai et al. Other studies have adopted the criteria for osteosarcoma presented by Cahan et al. [12] (widely used for the diagnosis of a radiation-induced sarcoma) and described a latency period of two years [6, 7]. In the present case, the latent period was five years and one month, which is also consistent with the definition. The patient’s initial malignancy was a SCC. Following radiation therapy, a pseudo-tumor was detected with no evidence of malignancy. Considering that undifferentiated spindle cell sarcoma is a rare disease, it seems appropriate to conclude that it was induced by radiation therapy in the present patient.
Table 1
Diagnostic reliability of radiation-induced malignancies according to Sakai et al. [8] (partially modified)
A radiation-induced pseudo-tumor develops earlier than sarcoma, with a median latent period of 79 months [13]. Although both sarcoma and a pseudo-tumor can be induced by radiation, it remains unclear whether a pseudo-tumor represents the precursor stage of a sarcoma [14]. A literature review of radiation-induced sarcoma showed that median age at diagnosis of radiation-induced sarcomas of the head and neck was 52 years (inter-quartile range, 21.5 years), with a mean latency between initial radiation therapy treatment and diagnosis of 11.1 years (range, 1.3-38 years) noted in one study [5], and 11.5 years (range, 6-17 years) reported by another [9].
The rates of occurrence of radiation-induced sarcomas in the head and neck range from 0.14% to 1.8% in the analyzed cases, including reports of external beam radiation therapy and LDR-ISBT [9, 10, 15]. The present case is the first reported instance of malignancy induced after HDR-ISBT in a patient treated at our hospital, and a search of the literature revealed no other report of radiation-induced malignancy development following that procedure. According to a retrospective study of radiation therapy for oral SCC, a radiation-induced pseudo-tumor is considered to be a rare complication, with a prevalence of only 0.02% [13].