Purpose
Uveal melanoma (UM) is the most common intra-ocular tumor in Caucasian adults and may derive from melanocytes within the iris (4%), ciliary body (6%), or choroid (90%), with a mean age-adjusted incidence of 5.1 cases per million per year [1, 2].
Historically, the first therapeutic approach to treat UM has been surgical removal of the eye, with obvious consequences in terms of function, cosmesis, and psychological impact on patients. First attempts to preserve the eye were performed in 1930 [3]. In 1985, the Collaborative Ocular Melanoma Study (COMS) introduced first uniform approach to perform interventional radiotherapy (IRT) procedure in a standardized way that allowed to demonstrate the equivalence of IRT with enucleation in terms of overall survival [4].
Since this milestone, several international guidelines have been issued with regard to several technical aspects of this procedure, which has become the mainstream therapy worldwide [5].
In particular, IRT may preserve both cosmesis and visual acuity (VA), which at 3 years after treatment usually reaches a stability in terms of VA loss [6].
In case of a recurrence, the main therapeutic approach is enucleation but in selected cases of marginal recurrences located at the equator (excluding posterior location of the recurrence and diffuse, or global recurrence), an IRT re-treatment may be considered [7]. However, nowadays, there is still a lack of consensus about the criteria to definitively assess UM response after IRT.
We present a collection of paradigmatic cases treated in our institution, and then discuss in detail the latest available evidence on the topic.
The objective of this article was to retrospectively review the imaging findings associated with uveal melanoma changes after brachytherapy, and to provide didactic cases.
Therefore, we included patients affected by uveal melanoma in different location (iris, ciliary body, choroid) and various morphology (dome-shaped, mushroom-shaped, or bilobated), and treated with either 106Ru or 125I IRT.
Material and methods
A series of 8 non-consecutive patients, diagnosed with UM and treated at our Interventional Oncology Center (IOC) with IRT according to INTERACTS guidelines, was presented [8].
All patients underwent an ophthalmic examination before plaque treatment and every six months after IRT in order to identify possible early recurrences. The examination included best correct visual acuity, slit amp biomicroscopy with photography, fundus photography, gonioscopy, ultrasonography, and indirect ophthalmoscopy.
Ultrasound examination was performed using high-frequency ultrasound transducer for the iris and ciliary body tumors, and B scan and A scan for posterior tumors. Tumor thickness was measured at the thickest portion of the tumor, and longitudinal and transverse diameters were measured. For the iris and ciliary body melanomas, in addition to tumor elevations and diameters, we routinely examined all 360° of the anterior segment. Other high-frequency ultrasound characteristics were evaluated, including tumor shape, scleral invasion, inner vascularization, and internal tumor reflectivity. A comprehensive imaging of longitudinal and transversal ultrasound scans were reported for each clinical case at baseline and after treatment to illustrate ultrasound regression pattern. When the ciliary body and choroid were involved, magnetic resonance imaging (MRI) was used to better demonstrate full antero-posterior extension of the lesion.
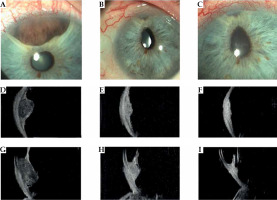
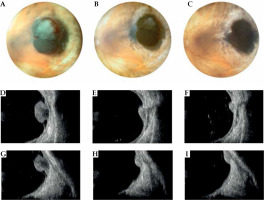
Fig. 1
Case 1. A) Slit lamp photograph reveals the iris portion of the pigmented iridociliary melanoma involving the anterior chamber of the eye at diagnosis. B, C) Slit lamp appearance at 1 and 3 years after ruthenium plaque showing a marked regression of the iris portion of the tumor. A radiation-induced cataract had been removed and an intraocular lens inserted; a posterior synechiae developed as a radiation effect. D-F) Transversal high-frequency ultrasound of the iridociliary melanoma before treatment and at 1 and 3 years after ruthenium plaque. G) Longitudinal high-frequency ultrasound of the iridociliary melanoma before treatment reveals its’ posterior extension into the ciliary body and the rupture of iris-pigmented epithelium. H, I) Regression of the ciliary-body tumor at high-frequency ultrasound, but the anterior chamber angle is involved
Patients were treated by 106Ru or 125I according to height of the lesion, using a 5-millimeter cut-off; for lesion below the cut-off, we used 106Ru and for lesions above the cut-off, 125I was used [9].
Data were retrospectively extracted within the frame of COBRA (consortium for brachytherapy data analysis) database to provide a visual atlas with relative timing of the correct assessment after IRT.
The cases presented in this paper were identified by a multidisciplinary team composed of two radiation oncologists (BF, VL) and two ophthalmologists (MMP, MGS). Another working group consisted of a radiation oncologist (CC), two ophthalmologists (CC, FB), a radiation therapist (PC), and a medical physicist (EP), performed a comprehensive review of the existing literature on the topic. Finally, four senior members, namely two radiologists (RI, CC), a radiation oncologist (LT), and an ophthalmologist (MAB) provided final approval to the manuscript.
Results
Case 1
Patient number 1 was a 58-year-old male patient with a pigmented iris and ciliary body melanoma on the left eye. On slit lamp physical examination, he presented dilated and tortuous episcleral blood vessels in the upper sector, and a nodular pigmented neo-formation placed between 10 and 1, occupying the iris stroma from the root, with an expansive growth in the anterior chamber to the corneal endothelium, as shown in gonioscopic image. Although the lesion involved the iridocorneal angle, the ocular tone was in the range of normal (15 mmHg).
During ultrabiomicroscopic examination, it appeared as a ciliary body melanoma with iris extension; the hyperreflective iris pigment epithelium was partially eroded by tumor. Internal reflectivity was low. It extended from the pars plana to the intermediate portion of the iris, with infiltration of the iridocorneal angle up to the corneal endothelium. Posteriorly, it displaced the lens. Its’ thickness was measured 5.4 mm, with the largest basal diameter of 13 mm. Based on these findings, a clinical diagnosis of the iris and ciliary body melanoma with anterior chamber invasion was made. The AJCC 8° edition stage was T2bN0M0. The patient underwent IRT with 106Ru CCB plaque (prescribed dose 100 Gy) in 2017. At 1-year post-IRT, the tumor showed partial remission; the pupil was irregular due to the presence of posterior synechiae. After 3 years, an ultrasound documented a remarkable reduction in tumor size, consisting in a flat area of iris pigmentation, and a ciliary body nodule with a thickness of 2.4 mm with increased acoustic reflectivity. The patient underwent cataract surgery; the best corrected visual acuity decreased from 20/25 at baseline to 20/63 at 1-year follow-up, and 20/200 at 3-year follow-up. The patient is still alive, with no evidence of recurrence.
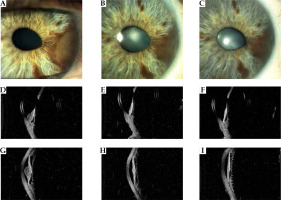
Fig. 2
Case 2. A) A nodular pigmented melanoma in the midportion and root of the iris producing a corectopia. B, C) Appearance of the tumor at 1 and 3 years after ruthenium treatment showing a regression of the tumor. D-I) High-frequency ultrasonography imaging revealing low internal reflectivity of the iris nodular tumor at diagnosis and reduction in thickness, and increase in internal reflectivity at 1 and 3 years follow-up. Note the intact pigment epithelium layer and no involvement of the ciliary body. After 1 year from treatment, the anterior chamber angle resulted free
Case 2
A 55-year-old male patient came to our attention with a history of excision of cutaneous melanoma. He had an iris nevus in his left eye that had changed over the last year. The best corrected visual acuity was 20/20 in both eyes. Slit lamp examination revealed a nodular pigmented mass of the iris, with irregularity of the pupillary foramen. At high resolution ultrasound biomicroscope, the tumor appeared as a hypoechoic thickening of the iris stroma with intact pigmented epithelium layer, measuring 1.95 mm in thickness and 5 mm in largest basal diameter.
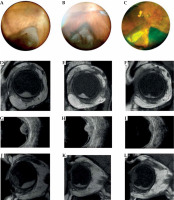
Fig. 3
Case 3. A-C) Fundus photograph of a choroidal melanoma located inferiorly at diagnosis and at 1 and 3 years after treatment. Note that the images do not allow to locate the anterior margin of the tumor. D-I) MRI and ultrasound images showing the transversal extension of the tumor at diagnosis and at follow-up. J-L) Sagittal magnetic resonance imaging in a T1-weighted image at diagnosis and at follow-up. MRI is particular useful in measuring longitudinal diameter in cases of choroid melanoma with anterior extension into the ciliary body and iris
The diagnosis was of iris melanoma, and IRT with 106Ru CCD (prescribed dose 100 Gy, dose to the sclera 260.4 Gy) was performed in 2018. No metastasis was found in a systemic work-up.
One year after the treatment, high-resolution ultrasound biomicroscopy (UBM) of the iris showed a partial regression with 1.24 mm thick. The patient is still alive, with no evidence of a recurrence.
Case 3
A 65-year-old woman was referred to our hospital with a chief complaint of visual field defect in her left eye. Her corrected visual acuity was 20/20 in both eyes. Slit lamp examination showed a large darkly pigmented mass in the inferior quadrant. B scan and UBM examination were performed and revealed a tumor of choroidal origin extended from the root of the ciliary body to the equatorial portion of the bulb, with a domed appearance, a homogeneous acoustic structure, and a medium-low reflectivity. It was associated with perilesional retinal lift with signs of neo-vascularization. The lesion had a thickness of 6 mm (A and B scan) and 10.7 mm of basal transversal diameter. Due to the ciliochoroidal location, B scan and UBM ultrasound examination were not a useful tool for measuring antero-posterior diameter. MRI measured a longitudinal diameter of 18 mm. The diagnosis was ciliochoroidal melanoma stage T3N0M0, and the patient underwent IRT with 125I plaque COMS 18 (prescribed dose 85 Gy) in 2013. Follow-up imaging was performed using both ultrasound and MRI, and showed a progressive regression of the tumor. The best corrected visual acuity at 3 years’ follow-up was 20/32. The patient is still alive, with no evidence of a recurrence.
Case 4
A 77-year-old male patient was diagnosed with a choroidal melanoma during a routine eye examination. The tumor measured 3.84 mm in thickness and 16.6 mm in largest basal diameter (stage T3N0M0); it had a bilobed appearance and was located in the nasal quadrant in the juxtapapillary area. Baseline best corrected visual acuity was 20/20. A treatment plan for bilobed melanoma was arranged, and the patient underwent IRT with 106Ru CCC plaque (prescribed dose 100 Gy, dose to the sclera 603.8 Gy) in 2018. Respectively, at one and three years from the treatment, the main dome had a thickness of 2.8 mm and 2.5 mm, and high internal reflectivity at B scan ultrasound examination. Minor dome showed a slower regression, and was treated with adjuvant transpupillary thermotherapy (TTT).
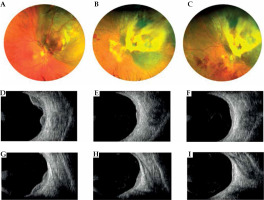
Fig. 4
Case 4. A) Fundus photograph of a bilobated choroidal melanoma located nasally to the optic disc. B, C) Regression of the tumor at follow-up and appearance of radiation and optic retinopathy. D-I) Transversal and longitudinal B-scan ultrasound clearly showing the bilobed morphology of the choroidal melanoma at diagnosis and tumor regression after ruthenium plaque
The patient developed radiation maculopathy and was treated with intravitreal injection; best corrected visual acuity after 3 years from the treatment was 20/400. The patient is still alive, with no evidence of a recurrence.
Case 5
A 42-year-old male underwent IRT with 106Ru CCD plaque (prescribed dose 100 Gy) in 2012 for a pigmented choroidal melanoma in his right eye located in the temporal quadrant. The pigmented choroidal melanoma measured 4.65 mm in height and 11.30 mm in largest basal diameter (stage T2N0M0). Baseline best corrected visual acuity was 20/20; after one year follow-up, the tumor had regressed to 2.6 mm, and visual acuity decreased to 20/32. 36 months after the treatment, visual acuity decreased to 20/100, and radiation maculopathy was diagnosed, treated with intravitreal injection and laser therapy. After 3 years from the treatment, the tumor thickness had regressed to 2.1 mm. The patient is still alive, with no evidence of a recurrence.
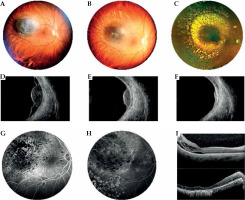
Fig. 5
Case 5. A) A mildly pigmented choroidal melanoma located temporally to the macula at diagnosis. B) Partially regression of the tumor at 1 year after interventional radiotherapy. C) Excellent regression of the tumor at 3 years after interventional radiotherapy. Laser treatment carried out for the development of radiation retinopathy. D) B scan ultrasonography showing dome-shaped morphology of the tumor, low internal reflectivity, and retinal detachment at diagnosis. E, F) B scan images showing tumor regression and resolution of retinal detachment after 1 and 3 years from interventional radiotherapy. G, H) FAG reveals vascular changes before and after retinal laser treatment with fluorescein staining in the photocoagulation area. I) OCT B-scan section shows (1) cystic intraretinal fluid and subretinal exudation before laser treatment; (2) after laser treatment, a large chorion-retinal atrophy present in the same area
Case 6
A 44-year-old woman was referred to the Ocular Oncology Service because of a growth of an amelanotic choroidal nevus that for the 4 previous years had been stable. The patient had no significant past medical history; best corrected visual acuity was 20/20 in both eyes. Ophthalmoscopy examination showed an amelanotic choroidal lesion with a pigmented base, a dome-shaped configuration and prominent intrinsic vessels, located in the inferotemporal hemisphere at the equator in her right eye.
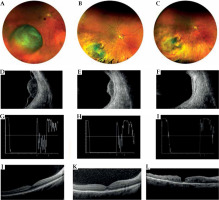
Fig. 6
Case 6. A) Fundus photograph of a large amelanotic choroidal melanoma located infero-temporally. B, C) Excellent regression after iodine-125 interventional radiotherapy. D) B scan transversal image showing dome-shaped morphology, low internal reflectivity, and the presence of retinal detachment before treatment. E, F) Tumor regression at 1 and 3 years follow-up. Resolution of retinal detachment. G) A scan ultrasonography at diagnosis showing 6.8 mm in thickness (distance between the arrows). Low internal reflectivity between the anterior and posterior peaks. H) At 1 year follow-up, the scan shows reduction of thickness (3.7 mm) and persistence of low internal reflectivity. I) At 3-year follow-up, A scan image shows decrease in thickness (2.9 mm) and increase of internal reflectivity. J-L) OCT B scan section shows at baseline, at 1, and 3 years after interventional radiotherapy the onset of intraretinal cystic edema, intraretinal lipidic exudation, and macular hole
At A-B scan ultrasonography, the tumor had a dome-shaped choroidal morphology, low internal reflectivity, regular internal structure, internal blood flow (vascularity), associated serous retinal detachment, and measured 6.86 mm in height and 15 mm in largest basal diameter. No evidence of metastatic lesions was present (stage T3N0M0). The patient was treated with COMS 18 125I plaque (prescribed dose 85 Gy, dose to the sclera 274.1 Gy) in 2018. After 1-year follow-up, the tumor had regressed to 4 mm in thickness, and after 3 years to 2.2 mm. Visual acuity was 20/20 at baseline, 20/25 at 1-year follow-up, and decreased to 20/200 at three years because of radiation maculopathy development. The patient is still alive, with no evidence of a recurrence.
Case 7
A 75-year-old man was referred to our Ocular Oncology Service following a chance discovery of a pigmented lesion in his right eye. Best corrected visual acuity was 20/32 in both eyes. On ophthalmoscopic examination, he presented a deeply pigmented, mushroom-shaped choroidal lesion, located temporal to the macula in the right eye, with a breakthrough Bruch’s membrane, retinal detachment, and localized vitreous hemorrhage. At B-scan ultrasonography, the lesion showed low internal reflectivity, and measured 6.2 mm in height and 9.8 mm in largest basal diameter; it was associated with signs of internal vascularization and serous retinal detachment. There was no evidence of any other primary/metastatic lesions on systemic evaluation. The stage was T2N0M0.
The patient was treated with COMS18 125I plaque (prescribed dose 85 Gy) in 2011. At one-year follow-up, choroidal melanoma had remained regressed at 3.7 mm thickness, and at a three-year follow-up, the tumor had regressed to 2.9 mm in thickness and presented increased reflectivity at ultrasound examination. At that time, best corrected visual acuity had decreased to 20/200 in the affected eye, secondary to the development of radiation maculopathy. Systemic evaluation showed no evidence of systemic metastases. The patient is still alive, with no evidence of a recurrence.
Case 8
A 75-year-old woman came for observation because of an appearance of a pigmented lesion in her right eye. External examination OD revealed nasally at 3 o’clock, two pigmented episcleral masses, measuring 2 mm in basal diameter and 1 mm in thickness, associated with dilated and tortuous episcleral vessels. Slit lamp biomicroscopy and gonioscopy OD revealed a pigmented nodule of the iris. Ultrasound biomicroscopic image through the ciliary body region OD confirmed the presence of a thin epibulbar ipoecogenic tissue mass corresponding to extrascleral tumor extension. The ciliary body tumor measured 4.30 mm in thickness, 11.45 mm in transversal diameter, and 8 mm in longitudinal diameter, and extended anteriorly with infiltration of the iris and the angle. The diagnosis was primary uveal melanoma of the ciliary body with iris infiltration and nodular extrascleral extension. The stage was T2dN0M0.
Fig. 7
Case 7. A) A pigmented mushroom-shaped choroidal melanoma at diagnosis. B, C) Regression of the tumor at 1 and 3 years follow-up after iodine plaque. Note the chorioretinal scar around the tumor and increase in pigmentation after treat- ment. D-G) B scan ultrasonography of the mushroom-shaped melanoma at diagnosis; the lesion shows typically higher reflectivity near the tumor apex and hollowness of the tumor base. E-I) B scan transversal and longitudinal images at 1- and 3-year follow-up
The treatment consisted of IRT with CCD 106Ru (prescribed dose 100 Gy) in 2009. On subsequent 1-year follow-up examination, there was a complete disappearance of the iris and episcleral nodule, and a partial remission of the ciliary body tumor to the thickness of 2.9 mm. At 3 years follow-up, the ciliary body tumor had regressed to 2.5 mm in thickness, and presented increased reflectivity at ultrasound examination. Best corrected visual acuity was stable to 20/25. The patient is still alive, with no evidence of a recurrence.
Discussion and conclusions
Following IRT, it is widely known that some UMs respond faster than others [10]. US assessment is the main and most commonly used diagnostic strategy to monitor UM regression; however, in the recent years, some authors have started to use MRI in addition to monitor UM response to IRT [11]. Early studies highlighted that regression of tumors rarely results in a flat and depigmented scar [12].
The first attempts to study UM responses emphasized that possible outcomes following IRT may be categorized into four main patterns according to changes in UM height: type D (decrease), type S (stable), type I (increase), and type M (miscellaneous). Noticeably, the most heterogeneous category (type M) includes clinical situations, in which in some cases, the response remains stationary after an initial shrinking (maximum shrinkage reported: 8 mm in 29 months) and then, after some time, may start shrinking again (second regression may start at 8 to 60 months after IRT, and the magnitude of the shrinkage may be in some cases dramatic) [13].
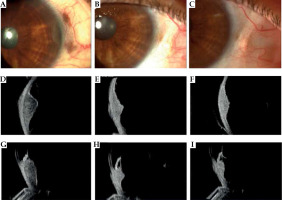
Fig. 8
Case 8. A) Slit lamp photograph at diagnosis reveals a pigmented iris nodule in the anterior chamber of the eye, two small pigmented episcleral nodule of extrascleral extension, and sentinel vessels. B, C) At 1 and 3 years follow-up after ruthenium plaque, note the disappearance of the iris pigmented nodule and extrascleral extension. D-F) Transversal high-frequency ultrasound imaging showing the iris and ciliary body melanoma before and after treatment. G) Longitudinal high-frequency ultrasound imaging of the ciliary body melanoma revealing its’ anterior extension into the iris root, anterior chamber angle, and posterior into the choroid. H, I) Note the decrease in tumor size after treatment and disappearance of the extension in the anterior chamber
More recently, other authors confirmed that regression patterns might actually be divided into 4 different outcomes, such as ‘decrease’, ‘stable’, ‘increase’, and ‘other’. The ‘decrease’ pattern was found to be the most common occurring in 43% of all cases. Interestingly, the second most common outcome was ‘other’ accounting for almost 40%, and authors have proposed some sub-categories, such as ‘decrease-stable’ (16%) and ‘zigzag’ (10%). Remarkably, authors report that regression pattern of the thickness is slower when compared to regression pattern of the area; additionally, regression patterns of the thickness versus the area may even be discordant in up to 1/3 of cases. Another extremely interesting finding emerged considering tumors with an unbroken, indeterminate, and broken Bruch’s membrane; in fact, increasing proportions regressed according to the ‘decrease’ pattern [14]. Other researchers, who have similarly focused on regression patterns, have highlighted that tumor regression rate raises with increasing tumor height and slows down with increasing of largest basal diameter [15].
Focusing on time, some authors have demonstrated that usually the largest decrease of tumor size is expected to occur within 12 months after IRT [16]. However, a proper follow-up period to definitively assess the response should be extended up to 3 years according to other experiences [17]. Several researchers have even proposed an early time cut-off of 6 months to identify possible insufficient tumor regression or tumor progression [18].
More in detail, the speed of decrease of UM height after IRT starts with an initial rate of approximately 3% per month. Most of the tumors do not regress entirely; rather, their height stabilizes with a constant value (on average 61% of the initial height) after approximately 18 to 24 months [19]. Initial tumor regression rate has been associated in some studies with a prognostic value, and faster regression rates tend to be associated with higher incidence of metastases [20].
Such association has not been confirmed in other series, where a regression rate at 6 months was sub-divided into three different patterns, including ‘slow’ = 0.02 mm per month, ‘medium’ = 0.32 mm per month, and ‘fast’ = 0.67 mm per month. In this series, no association metastatic rate and response rate in medium-sized UM were observed [21].
Several authors have shown that thicker uveal melanomas present a steeper and more pronounced reduction in terms of thickness following IRT. In particular, after first phase, the regression curve becomes similar for tumors with different thicknesses [22].
More recently, other researchers have identified a different model of tumor response, which can be modeled by a negative exponential function for the first 7 years after treatment, depending on initial height, except for those less than 2.5 mm [23].
A recent systematic review and meta-analysis highlighted that the efficacy of 106Ru in controlling uveal melanomas decreases with an increase in tumor thickness [24].
When comparing different radiotherapy techniques, among them, there is evidence in literature that IRT results in a faster tumor regression compared to stereotactic radiotherapy (gamma knife or linear accelerator) [25]. There is additional evidence supporting that IRT allows to obtain a faster response compared to proton beam therapy [26].
Ocular IRT utilization in clinical practice should be encouraged because available data support its’ use both in terms of efficacy and safety. The possible causes for its’ underuse across different centers have been a matter of debate, and several reasons have been highlighted, including specific areas of interest in the field of national guidelines, education, research, and communication with patients and colleagues of other specialties [27].
There are also other parameters, which should be taken into account when evaluating UM response, including decreased intrinsic tumor vascularity, decreased tumor-related sub-retinal fluid, increased pigmentation, specific changes in orange pigment lipofuscin, resolution of drusenoid retinal pigment in epithelial detachments, and increase in internal reflectivity on ultrasound. These parameters are especially important for situations, when a single-measure change in measured thickness does not comprehensively describe findings associated with regression, such as the case of iris melanomas [28, 29].
Another point of paramount relevance is the use of intra-operative ocular ultrasonography to verify the correct position of the plaques in patients treated uveal melanoma [30].
When it comes to considering the relationship between tumor response and genes, there are contradictory experiences in the literature [31, 32]. In fact, some researchers have pointed out that there is no distinct association between gene class type in UM after IRT at 3 and 6 months, when considering baseline tumor height [33]. However, other authors have found that class 1 UM tumors tend to regress more rapidly than class 2 tumors in the first 6 months after IRT [34]. Various investigators have even identified a distinct subset of class 1 UM (3%) that experience a very rapid tumor regression associated with transient tumor inflammation and uveitis underlying an immune-modulated mechanism [35].



