Purpose
Radiation is an essential modality of therapy that is used to treat patients with locally advanced gynecologic (GYN) cancers. Patients are typically treated with pelvic chemoradiation therapy followed by high-dose-rate (HDR) brachytherapy [1,2,3,4]. For these patients, brachytherapy is an important part of their treatment and can improve patient outcomes [5,6]. Normally, these patients are treated with intracavitary brachytherapy, where the applicator is placed directly into the cervix. However, for patients with larger tumors with distal vaginal involvement, bulky tumors with poor response, vaginal stenosis, which does not permit intracavitary treatment, or recurrent disease, interstitial brachytherapy (ISBT) is necessary for satisfactory coverage [7,8,9]. In ISBT, a transperineal template is used through which several hollow needles are directly inserted into the tissue to deliver dose more selectively to the tumor [10].
Studies have shown that interstitial brachytherapy is highly effective for locally advanced gynecologic cancers, with a 2-year locoregional control ranging from 51.3% to 93% [11,12]. Another study found that ISBT had similar overall survival and local control when compared to intracavitary brachytherapy despite the ISBT group including patients with larger tumors [13]. Unfortunately, patients who are treated with interstitial brachytherapy are typically treated with a high-dose to a large volume of tissue, which increases the risk of delayed toxicity [14,15]. These toxicities can prolong recovery and could potentially be fatal. In particular, vesicovaginal or rectovaginal fistulas present a significant risk because they are extremely difficult to surgically repair and can significantly affect a patient’s quality of life. Previous studies have shown that several factors including dose to 2 cm3 (D2 cc) of the rectum, smoking, comorbidities, and prior abdominal surgeries are predictive for fistula formation in patients treated with traditional intracavitary brachytherapy and interstitial brachytherapy [16,17,18,19,20,21,22,23]. However, risk factors specifically for fistula formation have not been studied in patients treated with interstitial brachytherapy due to small number of patients treated by this modality. The purpose of this study was to determine risk factors for fistula formation with ISBT. These risk factors could be used to identify patients at a high-risk for fistula formation and subsequently change clinical decisions and treatment planning to prevent toxicities.
Material and methods
Patients
After Institutional Review Board (IRB) approval, we analyzed patients treated with ISBT for GYN cancers from years 2011 to 2017. We found 44 patients who had been treated with interstitial brachytherapy. Demographic information, treatment regiments, toxicities, health information, survival, and recurrence data were collected through a review of the electronic medical records. Patients had a variety of gynecologic cancers including vaginal, endometrial, and cervical cancer. Three patients included in the study had recurrent cancer and 3 were treated with radiation previously.
Treatment
All patients were treated with ISBT following ABS guidelines, with 3-dimensional CT-volumetric planning as previously described [8,24]. Patients were treated with one insertion and five BID fractions of 4.5 to 5.5 Gy each to the clinical target volume. CTV contours were determined based on fiducial marker, clinical extent at the time of implant, cross sectional CT imaging at the time of implant, and cognitive fusion with preimplant MRI if available.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 24 (IBM Corps). Descriptive statistics reported mean, median, range, and standard deviation. Local control (LC), regional control (RC), overall survival (OS), and progression-free survival (PFS) were estimated using the Kaplan-Meier method. LC and RC were calculated in months from the start of radiation therapy to the date at which tumor progression occurred. OS was calculated in months from the start of radiation therapy to the date of death from any cause or censorship, which was defined as the last date of contact. PFS was calculated from the start of therapy to any recurrence or death. Univariate analysis was analyzed with the χ2 test. Univariate analysis looked at numerous prognostic factors including bladder and rectum invasion, and whether patients had biopsies or not. Bladder and rectum invasion were confirmed through either imaging or biopsy. Biopsies were defined as a biopsy of the vagina or treatment site post-treatment. Lastly, fistula formation was defined as an abnormal connection between the vagina and the bladder, ureters, rectum, or sigmoid colon and confirmed through either radiologic imaging or clinical evidence. Univariate logistic regression and area under the curve analysis were also performed to verify our results. Multivariate analysis was performed through MANOVA. Statistical significance was determined as p < 0.05.
Results
Forty-four patients were included in the study. Tables 1-3 describe patient characteristics, radiation treatments, and dosimetric data.
Table 1
Patient demographics and cancer characteristics
Table 2
External radiation treatment modality
| Number treated with IMRT (%) | 32 (72.7) |
| Number treated with 3D conformal (%) | 9 (20.5) |
| Number treated with EBRT at OSH (%) | 4 (9.0) |
| Number treated with only ISBT (%) | 1 (2.3) |
| EBRT fractionation, Gy/fx | 1.8-2.0 |
Table 3
Radiation therapy treatment and dosimetric data
Based on univariate analyses, age ≥ 60 years, Hispanic ethnicity, bladder involvement, rectal D2 cc ≥ 70 Gy, and whether patients had post-radiation biopsies or not were predictors for fistula formation. These factors were confirmed through univariate logistic regression. Table 4 summarizes the p-values from the χ2 univariate analysis for fistula formation and the number of patients within each sub-group with fistula formation. For this analysis, we included patients who had fistula associated with recurrence and it is possible that recurrence may have been partially responsible in the etiology of these events. However, we analyzed many possible factors associated with fistula formation because we believe that some factors are independent of recurrence and can be used to help predict patients at a high-risk for fistula formation.
Table 4
Statistical significance from univariate analysis of risk factors for fistula formation
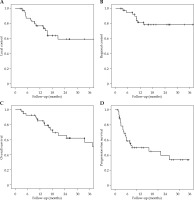
Median and mean follow-up time were 16.9 months and 23 months, respectively (range, 4.0-68.8 months). Table 5 and Figure 1 summarize the survival data including LC, RC, PFS, and OS.
Table 5
Survival data
| Survival | Mean (months) | 1-year | 2-year |
|---|---|---|---|
| LC | 43.5 | 76.90% | 59.30% |
| RC | 55.8 | 81.40% | 78.20% |
| OS | 43.1 | 85.00% | 62.00% |
| PFS | 33.9 | 58.50% | 49.90% |
Observed toxicities are summarized in Table 6. Of the 44 patients, 8 (20.5%) had grade ≥ 3 GI or GU toxicities. Nine (20.5%) of these patients had fistula formation with 1 vesicovaginal fistulas, 6 rectovaginal fistulas, and 2 with both. One patient had a fistula as a complication due to a hematoma. Of the 8 (18.1%) remaining patients, 6 had fistula formation with concurrent recurrence, thus only 2 (4.5%) patients had fistula formation definitively due to radiation treatment; however, all 8 patients were used in the causality analysis. These patients were managed either surgically through options like loop colostomies or symptomatically with the hope of the fistulas resolving without surgery. Of the 9 patients with fistulas, 3 were managed medically and 6 surgically. Three of these patients had grade 2 fistulas and 6 patients had grade 3 fistulas.
Discussion
Patients with advanced GYN tumors may require template-based interstitial brachytherapy boost in addition to standard external beam radiation therapy, though in some cases, interstitial brachytherapy can be monotherapeutic. Although ISBT can improve outcomes, the increased dose can lead to a greater risk of complications. Few studies [16,25] have examined predictors for severe toxicity following template-based ISBT for advanced GYN tumors. From our literature search, no studies have specifically looked at fistula formation after ISBT. This study attempted to define factors that lead to fistula formation for 44 patients with locally advanced gynecologic cancers treated with template-based HDR interstitial brachytherapy.
Our univariate analysis identified age ≥ 60 years, Hispanic ethnicity, D2 cc to rectum ≥ 70 Gy, bladder involvement, and whether patients had post-radiation biopsies or not as predictors for fistula formation (p = 0.03, 0.02, 0.02, 0.01, 0.04, respectively).
Although few studies have investigated predictors for fistula formation after ISBT, many studies have looked at predictors for fistula formation in traditional intracavitary brachytherapy and external beam radiotherapy. In contrast to our findings, Lebioda studied predictors of rectovaginal fistula formation in patients treated with LDR brachytherapy and found that age was not a predictor [19]. Similarly, Biewenga et al. found that age was not a predictor of vesicovaginal or rectovaginal fistula formation in patients with IVA cervical cancer [23]. Interestingly, another study that examined surgical treatment of rectovaginal fistulas postulated that age could affect tissue quality, which could lead to an increased risk of fistula formation [26].
Few studies have investigated race as a predictor for fistula formation. One study published in 1995 found that African American and Caucasian patients with stage IB cervical carcinomas treated with AP-PA radiation therapy were at higher risk for GI complications compared with Hispanic patients with the same stage of the disease and treatment [27]. There were no differences in weight or anterior-posterior separation between the groups. In contrary, our patient cohort found Hispanics more at risk for fistula formation in comparison to other races.
D2 cc to the rectum has been well established as a risk factor for GI toxicities and fistula formation. Lee and Viswanathan identified the D2 cc of the rectum as a predictor for rectal toxicities in patients treated with image-guided ISBT, similar to our study [16]. Georg et al. looked at many different parameters in dose-volume histograms and their abilities to predict late toxicities in patients treated with EBRT and brachytherapy. He identified D2 cc and D1 cc of the rectum as strong predictors for rectal toxicity [21]. Similarly, Kasibhatla et al. [16,25] studied predictors for GI toxicities in patients treated with EBRT and ISBT for advanced and recurrent GYN cancers. His study found that the 3-year risk of rectovaginal fistulas was significantly higher in patients who received a cumulative dose > 76 Gy to the rectum. Similarly, the EMBRACE study found that patients with a D2 cc to the rectum ≥ 75 Gy were at a 12.5% risk of fistula formation compared to 0-2.7% risk for lower doses [22]. We found the cut off rectal D2 cc dose that correlated with a significant incidence of fistula to be 70 Gy, which is closer to what has been reported by Lee et al. at 72 Gy [16,25]. Like the previous authors, we also found D2 cc of the rectum to be a predictor for fistula formation, since the majority of patients with fistula had rectovaginal fistulas.
Several studies have shown that bladder involvement is a risk factor for fistula formation [17,28,29,30]. Sun et al. reported that for patients with locally advanced cervical cancer, anterior tumor necrosis from bladder invasion was associated with vesicovaginal fistula formation [28]. Additionally, Moore et al. studied patients with cervical cancer that had invaded the bladder and found that 11 out of 23 patients had vesicovaginal fistulas. They concluded that patients with stage IVA cervical cancer that had invaded the bladder were at an extremely high-risk for fistula formation [17]. Hata et al. hypothesized that this may be due to rapid reduction in tumor volume that leads to fistula formation [30]. From this perspective, vesicovaginal fistulas could be a result of tumor response to treatment. We also hypothesize that patients with bladder invasion have tumors more likely to be aggressive and consequently are treated with a higher dose of radiation, which in general could predispose to more toxicity.
Lastly, we identified one study that showed post-radiation biopsies to be associated with fistula formation. Feddock et al. performed a retrospective study on the impact of post-radiation biopsies on patients with cervical cancer. Of the 89 patients who underwent invasive biopsy, 9 subsequently developed fistula. It was concluded that post-radiation biopsy was a risk factor for fistula formation [31]. It is possible that post-radiation biopsies are indicators for recurrence. In this scenario, tumor recurrence, rather than the biopsy itself, could cause tissue damage, which could lead to fistula formation. On the other hand, the presence of radiation changes or necrosis, which appear similar to recurrence, may require biopsy causing further trauma precipitating a fistula.
Other studies have identified additional factors that increase the risk of fistula formation. Murakami et al. investigated gynecologic malignancies treated with ISBT and identified re-irradiation and vaginal D2 cc as predictors of vaginal ulcers, which could develop into fistulas [32]. Moore et al. found that smoking, bladder, and rectum invasion were associated with patients with fistula formation. 56.5% of the study population of Moore et al. was found to be smokers, compared to 7 out of 44 patients (15.9%) in our study. This difference could explain why our study did not find smoking as a risk factor for fistula formation [17].
Limitations of our study include the retrospective nature of the study and the heterogeneity in the study population, since patients with primary and recurrent cervical, endometrial, and vaginal cancers were included and patients were not all treated with the same EBRT techniques. Additionally, the sample size was small, with 44 patients having had a limited follow-up period. Lastly, since only 8 of 44 patients had fistula included in analysis, the small sample size could impact the χ2 analysis. Regardless, we were able to identify some risk factors for fistula formation.
Conclusions
We identified prognostic factors that could predict fistula formation in gynecologic cancers after interstitial brachytherapy. In summary, the prognostic factors identified that predict fistula formation are age ≥ 60 years, Hispanic ethnicity, D2 cc to rectum ≥ 70 Gy, bladder involvement, and whether patients had post-radiation biopsies. Using these factors, we hope physicians will be able to identify patients at a higher risk for fistula formation after ISBT and make potential planning changes to reduce toxicities. However, there continues to be a need to further study patients treated by this modality. Studying the same principles through a multi-institutional study with a larger patient population could help confirm the factors we have identified in this study and indicate other factors that could predict fistula formation. This study is one step towards an eventual goal of identifying risk factors for fistula formation in patients treated with interstitial brachytherapy.