Purpose
The adrenal gland is a common site of malignant metastasis due to its’ rich blood supply, and is the 4th most common site of metastasis after lung, liver, and bone [1, 2]. With the development of diagnostic technology in recent years, diagnosis rate of adrenal metastases has continuously increased [3], and appropriate treatment strategies have received growing attention. Surgical resection is considered first-line treatment for this disease, especially for cases, in which primary cancer is well-controlled and the adrenal gland is the only site of a metastatic lesion [4, 5]. However, lack of functional preservation and incidence of complications related to adrenalectomy, such as hematoma, hypotension, pleural effusion, intercostal nerve injury, vein thrombosis, and infection, make surgery an ‘unsatisfactory’ treatment [6]. In addition, surgical resection cannot be performed in some patients due to age, surgical history, comorbid disease, or extra-adrenal cancers.
Radio-frequency ablation has been demonstrated to be an effective and well-tolerated treatment for adrenal metastasis [7-11]. The location of ablative range is limited by a complex relationship between the adrenal gland and surrounding organs. For that reason, the rate of complications (pain and hypertensive crisis) is considered high [10, 11]. Another mainstream curative treatment, radiation therapy, has been described in many studies. Ahmed et al. reported patients with adrenal metastases treated with stereotactic body radiotherapy, which provided a complete response (CR) + partial response (PR) of 91.7% in 24 patients with > 3 months of follow-up with serial computed tomography, with no radiation toxicity higher than third degree [12].
Another treatment approach, percutaneous brachytherapy, has been proven effective and safe for treating malignant tumors [13-15]. Computed tomography (CT)-guided radioactive iodine-125 (125I) seed implantation (CTRISI), which can be performed without surgery or general anesthesia, has attracted attention of clinicians in treatment of various malignancies, owing to its’ curative effect, minimal trauma, and few complications [16-20]. When implanted into the tumor permanently, 125I seeds deliver high radiation doses to the region of interest, with a sharp fall-off outside the implanted volume because of its’ low energy [21-25]. In addition, 125I seeds supply a continuous low-dose-rate of radiation over a long period, which was proven to be more effective than fractionated external beam radiotherapy [23]. Kishi et al. demonstrated the effectiveness and safety of permanent implantation of 125I seeds in the treatment of adrenal malignant metastases, showing favorable local control [26]. Seed implantation therapy has been studied in recent years, but there are few reports comparing CTRISI with traditional external radiotherapy.
Based on the above-mentioned results, we hypothesized that CTRISI might be an alternative approach for treating adrenal metastases. Therefore, tumor responses and complications in patients with adrenal masses treated with CTRISI in our institution were analyzed retrospectively.
Material and methods
Patients
This retrospective study was approved by an ethics committee, and written informed consent was provided by each participant. A total of 50 consecutive patients with adrenal metastases treated from August 2014 to April 2019 were enrolled. Inclusion criteria were as follows: 1) pathological diagnosis of adrenal metastasis, 2) well-controlled primary cancer and no additional treatment performed for adrenal metastases, and 3) surgical resection not performed due to poor physical performance, mediastinal lymph node metastasis, lack of patient consent, or other reasons. Since patients were already at an advanced stage, relative palliative radiotherapy was chosen, and 3D-CRT was applied instead of stereotactic body radiation therapy (SBRT) or volumetric modulated arc therapy (VMAT). VMAT has become a standard therapy worldwide. In this group of studies, 3D-CRT was mainly used as a palliative treatment for advanced oligometastases or extensive metastasis. Compared with SBRT treatment, it employs a lower biological dose and achieves relatively poor local control, but no significant differences in image verification and boundary control have been found. It has also been reported in the corresponding literature that SBRT can indeed be used as a non-invasive radical treatment for oligometastases, but this group of studies did not use it as a single-palliative treatment for oligometastases. Moreover, the literature reported that difference in local control rate between 3D-CRT and SBRT did not seem to be statistically significant [27].
Treatment
Among all patients, 19 and 18 lesions were treated with CTRISI and 3D-conformal radiotherapy (3D-CRT), respectively. For the CTRISI group, the planed dose was 110 Gy. Prescription dose chosen for the patients in the past was 110 Gy according to biologically effective dose for malignant metastasis of the adrenal glands in previous studies, which showed that more than 100 Gy was beneficial for local control. The dose that can be achieved by 125I source in previous studies ranged from 100-160 Gy, and the prescription dose selected for brachytherapy research in our country was also between 110-160 Gy. For safety reasons, 110 Gy was chosen for the treatment at that time. In the 3D-CRT group, the prescribed dose was 45 Gy in 25 fractions (180 cGy per fraction, once a day, 5 times a week). Monaco 5.11 system treatment planning system was applied after 5 mm thick slice CT scanning. Gross tumor volume (GTV) included macroscopic tumor on CT imaging, which was performed on a large aperture CT. A 5 mm safety margin was applied to form clinical target volume (CTV). An additional planning target volume (PTV) margin of 5 mm was further added to account for positioning inaccuracies. On a Synergy linear accelerator (Elekta, Stockholm, Sweden), 8 megavolt X-rays were delivered with 5-7 field irradiation. 90% isodose line completely covered PTV, and maximum dose did not exceed 10%. Organs at risk (OARs), including the liver, kidneys, stomach, spinal cord, and bowel were delineated: spinal cord (Dmax < 45 Gy), stomach (< 45 Gy), intestine (V15 < 120 cc), kidney (V12 < 55%), (V20 < 32%), and liver (Dmean < 30 Gy). Prescription dose of external radiotherapy for the control group was formulated by a radiotherapist in our hospital. General prescription dose of palliative 3D-CRT for adrenal metastases in our hospital’s radiotherapy department is based on a combination of international and domestic guidelines and previous studies (30-45 Gy). Patients selected for a retrospective analysis of this group received a 45 Gy prescribed dose of radiotherapy at that time. Symptomatic and support treatment was performed during radiotherapy. The other 14 patients who did not receive any treatment for adrenal lesions served as the control group.
125I seeds (Beijing Atom High Tech) were packaged in a cylindrical titanium body, with length of 4.5 mm, diameter of 0.8 mm, inside dimensions of 3.0 mm × 0.5 mm for silver column (adsorption of 125I, radioactivity: 0.6 mCi, average energy: 27.4-35.5 keV, half-life: 59.6 days, half layer: 0.025 mm of lead, antitumor effective radius: 1.7 cm), and wall thickness of 0.05 mm titanium for external shell. Before implantation, CT images with 5 mm section thickness were obtained for targeting the region of interest. A treatment planning system (TPS, Prowess Panther™) was used to create an individual treatment plan for each patient. TPS algorithm and coordinate system applied for brachytherapy dosimetry calculations [28] were based on TG-43 U1:
Where Sk is air kerma strength of source, Λ is dose rate constant, GL(r, θ) is geometry factor, gL(r) is radial dose function, and F(r, θ) is anisotropy function.
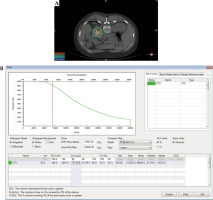
Treatment plan determined the dose of radioactive 125I seeds implanted and implantation site. After delineating the target area based on CT image and inputting the proposed prescription dose, we calculated dose and quantity of implanted sources through TPS. Then, a dose-volume histogram (DVH) was generated and isodose curves for different percentages were plotted. Simultaneously, coordinates of the brachytherapy applicator positioning could be displayed. We ensured that DVH achieved should result in D90 > prescribed dose and V100 > 90% (Fig. 1). The puncture site and path were determined to avoid unexpected complications, i.e., hemorrhage, pneumothorax, etc.
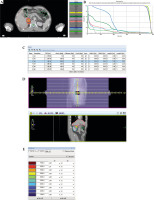
Fig. 1
Pre-planning of 125I brachytherapy. A) Site of the needle and seed and dose distribution on treatment planning system (TPS). B) Dose-volume histogram (DVH) for the tumor on TPS. C) General information of sources and other options in TPS
The patient was laid flat on a CT scanning bed, and after abdominal CT scanning, location and anatomical relationship of the lesion were determined. Positioning grid was used to confirm the puncture point was in line with the plan.
After local anesthesia, 18 G needles were inserted into the adrenal lesions under CT guidance. If the puncture path inevitably passed through the lung tissue, an artificial pneumothorax could be performed accordingly. The specific method was a puncture needle used to puncture to the edge of pleural cavity, but without entering the pleural cavity. Then, a small amount of air was injected after pulling out the occipital core and CT was re-checked. If a small amount of pneumothorax was found, the needle tip was located between the visceral pleura and parietal pleura. We continued to inject air until CT indicated there was no lung tissue on the puncture path. It should be noted that at the end of the operation, a puncture needle needs indwelling to extract gas from the chest cavity. Care should be taken to prevent formation of tension pneumothorax or subcutaneous emphysema, and a closed thoracic drainage tube should be indwelling when necessary. When the puncture needles reached the edge of the lesion, a CT scan was performed to ensure that the puncture site conformed to the treatment plan. An implantation gun was attached to the needle or applicator for implantation. 125I seeds were implanted while needles were exited simultaneously. Every seed was placed at a distance of 0.5-1.0 cm from each other (Fig. 2). CT scanning was performed to confirm distribution of 125I seeds. Within 1 month, TPS was applied again to verify the retention and placement of 125I seeds. If necessary, more 125I seeds were implanted into radioactively cold areas. If the patient had a cold zone within 1 month, re-calculation was performed according to target area changes on CT image, and the source radioactivity change was calculated based on the formula. After obtaining the result, the plan was re-designed and the corresponding activity was supplemented in that particle area. The activity calculation formula was as follows:
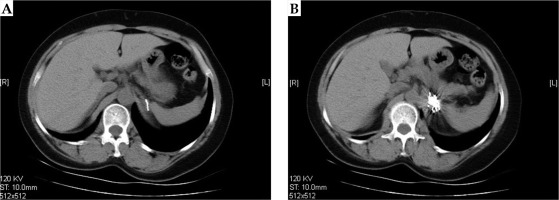
Fig. 2
Post-operative dose verification. A) Distribution of 125I seeds in the lesion. Isodose curve covers clinical target volume (CTV). B) dose-volume histogram (DVH) post-operation on treatment planning system (TPS)
Where A0 is initial activity, A is remaining activity, T1/2 is half-life of the source, and t is elapsed time.
During treatment procedure, symptomatic and support treatment were applied routinely and as needed.
Follow-up and safety evaluation
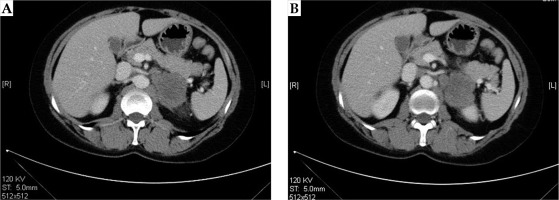
CT plain scan was performed at 6 weeks, 3 months, and 6 months after the treatment. The representative CT images before and after treatment are shown in Figures 3 and 4.
The maximum two vertical diameters of the lesion were measured to calculate the sum of diameters (Dsum). Tumor responses were evaluated by CT according to criteria for solid tumors [29]. Complete response (CR) was defined as complete disappearance of the tumor. Partial response (PR) was described as a ≥ 30% decrease in Dsum compared to baseline Dsum. Progression disease (PD) was described as a ≥ 20% increase in Dsum compared to the smallest Dsum. Stable disease (SD) was defined as neither a sufficient decrease to reach PR, nor a sufficient increase to reach PD compared to the smallest Dsum. Local control rate (LCR) included both CR and PR.
We compared the products (p-value) of two largest and perpendicular diameters within the tumor under CT examination before and after the procedure. Side effects during and after the procedure as well as complications during follow-up were recorded to evaluate the safety of treatment. A typical radiotherapy plan is shown in Figure 5.
Results
Patients characteristics
The characteristics of all patients are summarized in Table 1. There were 18, 18, and 14 patients with average ages of 54.33, 61.05, and 57.87 years in the CTRISI, 3D-CRT, and control groups, respectively. Bilateral metastatic lesions were found in one patient in the CTRISI group. In the CTRISI and control groups, 47.36% and 57.14% of the primary cancers were liver cancers. In the 3D-CRT group, 50% of the primary cancers were located in the lung. There were no significant differences in age, sex, lesion position, or primary cancer distribution among the CTRISI, 3D-CRT, and control groups.
Table 1
Characteristics of 51 patients with adrenal metastasis
Tumor responses
Tumor responses are summarized in Table 2. At 6 weeks, of the 19 lesions in the CTRISI group, 3, 11, and 5 lesions achieved SD, PR, and CR, respectively. In the 3D-CRT group, 10, 6, and 2 lesions showed SD, PR, and CR, respectively. At 3 months, 6, 12, and 1 lesion in the CTRISI group achieved SD, PR, and CR, respectively. In the 3D-CRT group, 1 tumor showed PD. At 6 months, 1 and 6 tumors showed PD in the CTRISI and 3D-CRT groups, respectively. There were significant differences in the rates of PD, SD, PR, and CR among the three groups at 6 weeks, 3 months, and 6 months (Table 2). From the follow-ups at 6 weeks, 3 months, and 6 months, the LCRs with CTRISI were 84.41%, 68.42%, and 57.89%, respectively, and those with 3D-CRT were 44.44%, 38.89%, and 22.23%, respectively. The difference between the LCRs in the two groups at 6 weeks was statistically significant (p < 0.05). In each group, therapeutic effects at the three follow-up times showed no statistically significant differences.
Table 2
Comparison of therapeutic effects of different treatments at different times
Complications
Complications in all groups are presented in Table 3. No severe complications or side effects, such as malignant hypertension, myelosuppression, or organ (kidney, lung, pancreas, etc.) injury occurred during the procedure or after the treatment. Over the treatment course, pain, radiation skin injury, hemorrhage, and pneumothorax were observed in 8, 1, 1, and 2 patients in the CTRISI group, respectively. In the 3D-CRT group, fever, pain, infection, radiation pneumonitis, radiation skin injury, and limb movement disorder were seen in 3, 7, 1, 1, 11, and 1 patient, respectively. Pain in all cases was relieved after analgesic treatments. One patient with pneumothorax was treated by thoracentesis and drainage. The drainage tube was placed after the exaction of gas and removed as the chest symptom disappeared. Other case with pneumothorax showed no obvious clinical symptoms, and no special treatment was given. In both cases, healing was confirmed by CT examinations 3 days later. The incidence of radiation skin injury differed significantly between the CTRISI and 3D-CRT groups.
Table 3
Comparison of complications between CTRISI and 3D-CRT groups
Discussion
Surgical resection has been reported to prevent recurrence of adrenal metastasis in 77-84% of patients [30, 31]. This rate is similar to LCRs achieved with CTRISI at 6 weeks and 3 months. However, at 6 months, the LCR rate with CTRISI decreased to 57.89%. Compared with 3D-CRT, CTRISI resulted in a significantly lower LCR at 6 weeks. At 3 months and 6 months, LCRs between these two groups were not statistically different. These results demonstrated that CTRISI treatment offers short-time efficacy for adrenal metastases, and for long-term control, only CTRISI treatment is not sufficient, achieving an unsatisfactory LCR. In addition, patients whose primary cancer was well-controlled and whose adrenal gland was the only site of metastasis, are eligible for surgery. For the patients included in the present study, surgical resection could not be performed due to poor physical performance, mediastinal lymph node metastasis, patient’s refusal, and other reasons. We believe CTRISI has critical significance in providing an additional treatment option for these patients. In addition, only one patient was observed to achieve CR after CTRISI at 6 months follow-up. Wieners et al. followed 19 patients with malignant tumors treated with interstitial brachytherapy, and of the two patients with adrenal metastases in their study, one patient achieved PR and the other died during 6-month follow-up [32].
Primary malignant tumors of the enrolled patients were lung or liver cancers. They showed no significant differences among the CTRISI, 3D-CRT, and control groups. There was no obvious screening of patients according to tumor volume in these three groups. Patients, who went to radiotherapy department received 3D-CRT treatment during the same time period, and patients who went to interventional radiology department, received CTRISI therapy. For follow-up observation, CT plain scanning was performed and referenced RECIST 1.1 technique was not used due to lack of tumor enhancement information. A p-value of 0.05 for comparisons of all age groups was noted, and although it was not < 0.05, it was very close. Among the three groups, the age in the CTRISI group was the youngest, and therefore, the influence of age on the efficacy and safety of this treatment requires further investigation.
In the present study, there was no incidence of severe complications and side effects, such as malignant hypertension, during the procedure or after the treatment. Over the treatment course, pain, radiation skin injury, hemorrhage, and pneumothorax were observed in 8, 1, 1, and 2 patients, respectively, after CTRISI treatment. In comparison, after radio-frequency ablation of adrenal metastases, more than 4% patients suffered from severe complications, including hypertensive crisis combined with acute myocardial injury and massive pneumothorax [10]. Additionally, after surgical adrenal metastasectomy, about 21% of patients experienced severe complications, including bleeding, pneumothorax, splenic tear, superior vena cava syndrome, inter-costal nerve injury, hematoma, hypotension, adrenal insufficiency, pleural effusion, prolonged ileus, deep venous thrombosis, and even death [6]. During the treatment course and up to 6 months after CTRISI, no severe complications were observed, indicating safety of CTRISI treatment.
There are several limitations of this study. First, the number of patients enrolled was small with only 18 patients included. Second, the time of follow-up was short at a maximum of 6 months. More cases and longer follow-up are needed for future survival analyses. Third, only localized treatment of adrenal metastases was applied in the current study, and the combination of this local approach with a systemic approach for tumor treatment should be investigated. Fourth, in patients with adrenal metastases in the lung and liver, systemic chemotherapy, including molecular target and therapeutics and immune checkpoint inhibitors, is the standard treatment, but the present study did not compare the efficacy of CTRISI with those of systemic chemotherapies [33, 34]. Fifth, in the current treatment of adrenal metastases, radiotherapy methods represented by SBRT and VMAT achieved therapeutic effects equivalent to surgery. Three-dimensional conformal radiotherapy has no advantages compared with SBRT and VMAT. Therefore, future research should select more optimized radiotherapy method as controls to verify the effect of seed implantation treatment.