Purpose
Surface mold, endocavitary, and interstitial brachytherapy (BRT) techniques have been used in the treatment of oral cavity mucosal cancers in the primary, adjuvant, or recurrent setting, alone or in combination with external beam radiotherapy [1, 2]. These allow for precise and highly conformal delivery of radiation to the mucosa with sparing of underlying bone in superficial disease, addressing dose inhomogeneity due to air-tissue interfaces.
Alveolar cancers are uncommon, comprising only 2.5-10% of oral cavity cancers in Western cohorts, but up to 40% in Indian cohorts, where tobacco use and betel nut chewing are prevalent [3]. Smoking, alcohol use, and tobacco use or betel nut chewing are the major risk factors; other less substantiated risk factors include poor oral hygiene, chronic trauma, such as from ill-fitting dentures, and periodontal diseases [4].
In a disease limited to the oral region, surface mold BRT using molds or prostheses are commonly used. With sinonasal extension, interstitial seeds alone or combined with surface mold BRT have been reported [5, 6]. Three-dimensional printing [7] and cast techniques [8-12] have been described for the fabrication of personalized applicators. These techniques require resources and expertise that may not be readily available, involve additional costs, and delay treatment starting.
Here, we report on the methods and outcomes of a simple approach to the fabrication of a personalized endocavitary BRT applicator in a case of a stage IVA maxillary alveolar squamous cell carcinoma (SCC) treated with definitive chemoradiation (CRT), followed by salvage surgery and adjuvant BRT.
Case presentation
A 67-year-old male presented with ill-fitting dentures and was found to have an indurated ulcer in the right maxillary alveolus. A panoramic radiograph showed an ill-defined radiopaque lesion suspicious for a malignant process. Computed tomography (CT) scan showed a 2.6 × 2.7 × 2.0 cm right maxillary alveolar ridge mass, with an involvement of posterior aspect of the right maxillary sinus, pterygoid bone, pterygomaxillary tissue, and buccal space, and prominent sub-centimeter right neck nodes. Biopsy of the lesion showed SCC.
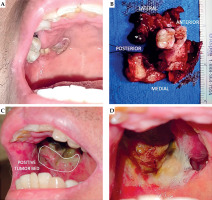
The patient had no comorbidities, and was not a smoker, tobacco or betel nut chewer, and alcohol beverage drinker. He was advised surgery and adjuvant CRT, but declined surgery and opted for definitive CRT. The primary tumor was treated to 70 Gy, with 5 mm expansion margins to 60 Gy, and bilateral neck level IB, IIA, IIB, III, IVA, and IVB to 56 Gy, given as sequential boost using intensity-modulated technique over 35 daily sessions in 7 weeks, with concurrent weekly cisplatin 40 mg/m2. Follow-up CT at four months post-CRT showed a 1.8 × 1.8 × 2.1 cm residual mass with minimal enhancement and mass effect, and no neck adenopathy. He was advised either surgery or chemotherapy, and opted for surgery due to tumor-related pain. At five months from completion of CRT, the patient underwent right posterior Brown class IIA maxillectomy. The involved alveolar segment was removed along with the lateral and posterior maxillary walls and hard palate (Figure 1A, B). Orbital floor was preserved, and no reconstruction or neck dissection was performed. Histopathology showed residual keratinizing SCC. Posterior and lateral margins were positive, while the rest of the margins were uninvolved. He was then referred for adjuvant BRT.
Material and methods
Applicator design
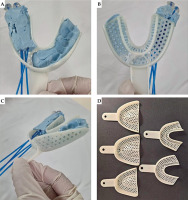
The personalized endocavitary applicator consisted of a dental impression plastic tray as the base, filled with vinyl polysiloxane (VPS) dental impression paste, to which two rows of flexible catheters were integrated on the right side, two catheters per row, with the catheters approximately 12 mm apart (Figure 2A-C). Left side that held the paste, to which the remaining dentition was imprinted, served as the docking site to ensure reproducibility of the placement.
Applicator fabrication
Proper size of the tray was determined by individual fitting. Left (docking) side was filled with VPS paste and a dental impression was made. Proximal (pharyngeal) ends of the catheters were secured with plastic buttons, and distal (oral) ends were tunneled into perforations in the tray, thus effectively protecting the catheters from the patient’s bite. Right side was then lined with a thin layer of the paste, to which the first row of catheters spaced 12 mm apart was secured. The same was done for the second row of catheters. Third row of catheters was initially planned; however, post-surgical inter-incisor opening of 25 mm as well as grade 4-5/10 post-surgical pain limited the height of the applicator. A third layer of the paste was laid on top of the second row of catheters, securing them in place. The assembled applicator was re-fitted into the patient, and excess bulk was removed to ensure comfortable fit.
Treatment planning
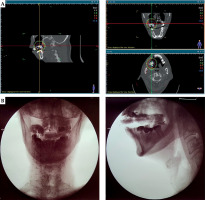
A 1 mm slice thickness CT with the applicator and CT markers in place was obtained. High-risk clinical target volume (HR-CTV) was delineated as the positive-margin tumor bed, while intermediate-risk CTV (IR-CTV) was defined as the entire tumor bed with 3-5 mm margins around HR-CTV. Fourteen 3.5 Gy high-dose-rate (HDR) fractions were prescribed to be given twice daily, 6 hours apart, in seven sessions weekly. Prescription doses were 3.5 Gy to HR-CTV, corresponding to a 5 mm depth from the mucosa, and 3.0 Gy to IR-CTV, corresponding to a 8-10 mm depth from the mucosa. Mucosal D2cc and D1cc were kept under 150% and 200% of the prescribed dose. Volumetric planning was preformed using BEBIG SagiPlan® treatment planning software. Per fraction, HR-CTV D90, IR-CTV D98, mandible D2cc, mucosa D2cc, and mucosa D1cc were 3.7 Gy, 2.4 Gy, 2.3 Gy, 5.2 Gy, and 6.5 Gy, respectively (Figure 3A). Assuming normal tissue repair and no tumor repair in the 6-hour interval, these doses corresponded to a total 2 Gy equivalent doses (EQD2) of 72.8 Gy (α/β = 10), 40.5 Gy (α/β = 10), 34 Gy (α/β = 3), 119.4 Gy (α/β = 3), and 172.9 Gy (α/β = 3), respectively.
Treatment delivery
The treatment was started at 26 weeks from completion of the prior CRT and 4 weeks post-surgery. The applicator was fitted into the right maxillary alveolar defect, and the dental impression from the intact left alveolus on the VPS paste helped ensuring good reproducibility. A 1 cm-thick pad of gauze was placed underneath to push the tongue inferiorly and away from the applicator. During the simulation and before every fraction, orthogonal radiographs were taken to verify and record applicator placements (Figure 3B). The patient was advised to maintain a firm bite on the applicator and depress the tongue throughout each fraction.
Results
The regimen was completed without technical difficulties or clinical complications. Good inter-fraction reproducibility of the applicator placement was observed and recorded, and CT simulation and re-planning was not required. Radiation mucositis peaked at grade 2 at two weeks post-BRT (Figure 1D). At four weeks, mucositis has diminished to grade 1, and pain to grade 3-4/10, which was managed with pregabalin, vitamin B complex as co-analgesic, and paracetamol-tramadol as rescue pain medication.
The patient remained disease-free based on physical examination (PE) and magnetic resonance imaging (MRI) at 3 months, and PE, chest radiography, and liver ultrasound at 6 months. The pain persisted, and the medications were maintained. At 7 months, PE revealed suspicious lesions in the adjacent buccal area. At 8 months, the lesions have involved the overlying skin. Biopsy confirmed a recurrence. The patient refused further treatment, and died within a month due to complications of acute biliary obstruction secondary to a pancreatic mass, a possible metastasis.
Discussion
As for other sites of the oral cavity, surgery is the preferred primary treatment for alveolar SCC [13]. Adjuvant radiotherapy with or without concurrent chemotherapy is administered in advanced disease. Here, we presented a case of advanced alveolar SCC that was managed with definitive CRT according to patient preference, with partial response but requiring salvage surgery. Adjuvant therapy was then indicated because of positive surgical margins. BRT was the preferred option due to prior irradiation and the presence of air-tissue interfaces in the target region. Therefore, an important dose inhomogeneity was expected if external beam radiotherapy was to be used [14].
In the presented case report, we described a simple approach to the fabrication of a personalized endocavitary BRT applicator using readily available materials. After discussing intra-operative and surgical pathology findings with the surgeon, the extent of tumor bed and location of positive margins could be accessed and encompassed entirely through intra-oral applicator (Figure 1C). Three-dimensional printing is not yet readily available locally and cast technique would be challenging, given surgical defect and communication with the maxillary sinus. Therefore, we fabricated a personalized applicator using a dental impression plastic tray that is available in different sizes and comes with perforations allowing the passage of flexible catheters (Figure 2D). A VPS paste was used that hardens to a rubbery consistency that is relatively resistant to tear, allowing patient comfort. Given different sizes and configuration of the tray, the approach can be utilized in alveolar or hard palate cancers.
A careful determination of CTV and adequacy of mouth opening were important to ensure that the cranio-caudal dimension of the applicator could be fitted and encompass the CTV. A peri-operative approach would be ideal to allow for examination and intervention under anesthesia [2]. However, in our case, there was limited residual tumor and involved margins were not expected. Therefore, an intra-operative referral was not made, and indication for adjuvant BRT was determined later. We were restricted by post-surgical limitation of mouth opening that precluded the addition of a third row of catheters. Therefore, we had to accept greater dose loading in a set of fewer catheters. On dosimetry planning, we found that the hyperdose sleeve (V200, 7 Gy) was mostly contained within the substance of the applicator, minimally reached the mucosa, and did not reach the mandible (Figure 3A). Balancing between adequate dosing to the CTV and minimizing doses to the normal tissue was very important, given that this was a re-irradiation. With careful planning of applicator design as well as execution and volumetric dosimetry planning, BRT is a safe and effective modality for re-irradiation, alone or in combination with external radiation and surgery [6, 15, 16].
The patient had a 6-month disease-free interval, with only grade 1-2 neuropathic pain. At 7 months, the patient developed local recurrence in the adjacent buccal mucosa that received high radiation doses, suggestive of tumor radio-resistance. Indeed, maxillary alveolar SCCs are known to be biologically more aggressive than their mandibular alveolar counterparts [17]. An Indian retrospective cohort of 642 cases of stage III-IV SCC of the gingivobuccal complex (mandibular alveolus, lower gingivobuccal sulcus, buccal mucosa, and retromolar trigone) reported 35.5% recurrence rate, a median post-recurrence survival of 2.7 months, and 2- and 5-year disease-free survival (DFS) rates of 63.8% and 53.3%, respectively [18]. In contrast, an Indian retrospective cohort of 110 cases of SCC of the maxillary alveolus reported 5-year overall survival rates of 65.6%, 56.7%, and 19.4% for stage III, IVA, and IVB disease, respectively; DFS was not reported for this cohort [17]. For unresectable recurrence, platinum agents and anti-PD-L1/PD-1 therapies are recommended options [13]. However, our patient opted for no further treatment.
Conclusions
A personalized endocavitary BRT applicator can be fabricated using readily available dental impression plastic trays and vinyl polysiloxane paste, allowing for lower costs and less treatment delays while ensuring patient comfort and convenience. The approach is suitable for alveolar and hard palate cancers in the primary or post-operative setting.