Introduction
Diabetes is a common metabolic disease with a chronic hyperglycemic state [1]. It is estimated that diabetes affects approximately 536.6 million people in 2021, representing 10.5% of the population aged 20-79, and type 2 diabetes (T2D) comprises approximately 90% of all diabetes globally [2]. Type 2 diabetes seriously affects multiple organs and can lead to various complications [3]. Diabetic encephalopathy (DE) is a central nervous complication of T2D, which displays Alzheimer’s disease (AD)-like pathology, with features of cognitive impairment and neuropathological changes [4]. Evidence suggests that hyperglycemia and insulin resistance can promote the development of AD-like changes in T2D [5]. Chronic hyperglycemia compromises the integrity of the blood-brain barrier, which plays a key role in maintaining central nervous system homeostasis [6]. Moreover, hyperglycemia also disturbs the microenvironment of neural stem cell niches and increases neuronal loss, consequently contributing to cognitive deficits [7]. Tau hyperphosphorylation in the hippocampus is a key pathological characteristic of AD pathogenesis [8]. Moreover, neuroinflammation caused by the release of proinflammatory cytokines has been demonstrated to have a close relationship with cognitive dysfunction and acts as a critical mediator in DE pathogenesis [9]. There is currently no effective treatment for DE [5]. Hence, there is still a pressing need to develop new effective strategies to prevent or treat DE.
Swertiamarin (SW) is a secoiridoid glycoside isolated from Enicostemma littorale and possesses various pharmacological properties, such as antioxidant, anti-inflammatory, anti-diabetic, and hepatoprotective activity [10]. Importantly, evidence suggests that SW has beneficial effects on dyslipidemia and glucose metabolism in a streptozotocin (STZ)-induced T2D rat model [11]. Research by Xu et al. demonstrated that SW could attenuate glucose intolerance and insulin resistance in obese mice [12]. Moreover, recent studies revealed that SW has a neuroprotective role in a mouse model of Parkinson’s disease [13]. Nevertheless, it is unclear whether SW has a neuroprotective role in diabetes-induced DE. The associated mechanism also remains unclarified.
Glycogen synthase kinase 3β (GSK3β) is a constitutive serine/threonine kinase implicated in mediating tau phosphorylation and insulin signaling in diabetic brains [14]. As a key tau kinase, GSK3β phosphorylates tau at many AD-related sites [15]. The phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway acts as a critical mediator in multiple biological processes. Importantly, upon activation, PI3K signaling can suppress G3K3β activity via Akt-mediated phosphorylation at Ser9 [16]. Activation of PI3K/Akt/GSK3β signaling in the hippocampus has been associated with improved cognitive impairment in T2D animal models [17].
Herein, we intended to explore the precise function of SW in T2D-induced DE and its associated mechanism using a rat T2D model. We speculated that SW could improve cognitive deficits, tau hyperphosphorylation, and neuroinflammation in T2D rats.
Material and methods
Animals
Male Sprague Dawley rats (5-6-week-old, 140-190 g) were purchased from Cavens (Changzhou, China) and housed under 12-h light/dark cycles with controlled temperature (22 ±2oC), humidity (50-60%) and free access to food and water. All experimental procedures were conducted following the Guide for the Care and Use of Laboratory Animals. Approval for the study was obtained from the Ethics Committee of Wuhan Myhalic Biotechnology Co., Ltd (approval number: HLK-20230224112; approve date: 2023.2.24).
Animal model establishment and drug treatment
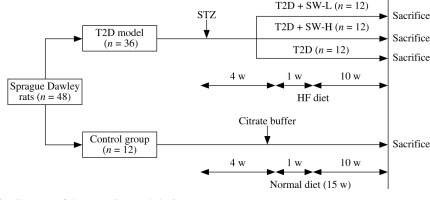
After one week of acclimatization, 48 SD rats were randomly assigned to the control group (n = 12) and T2D group (n = 36). To induce T2D, rats were fed with a high-fat (HF) diet (60% kcal fat; Trophic, Nantong, China) for 4 weeks and then received intraperitoneal injection of a single dose of STZ (35 mg/kg, dissolved in 0.1 M citrate buffer; MedChemExpress, Shanghai, China) after 12 h of fasting [18]. One week after STZ injection, animals with fasting blood glucose levels above 16 mM were confirmed diabetic. Then, the diabetic rats were further assigned to 3 groups (n = 12/group): the T2D group and T2D plus low-dose or high-dose SW (SW-L or SW-H) groups. In the T2D group, rats were fed with the HF diet for 10 weeks, and those in SW-treated groups were orally administered 25 mg/kg or 50 mg/kg SW (purity > 98%; MedChemExpress; dissolved in physiological saline) daily and fed with the HF diet for 10 weeks. SW was administered as a separate procedure from chow feeding. SW doses were determined based on previous reports [11, 19]. The control rats were fed with a normal diet for 15 weeks and received an injection of 0.1 M citrate buffer alone. Figure 1 shows a schematic diagram of the experimental procedure.
Measurement of blood glucose, insulin, and HOMA-IR
After fasting for 12 h, blood was collected from the retro-orbital sinus of rats under anesthesia and centrifuged at 3500 rpm for 10 min at 4°C. Fasting blood glucose level was determined using a commercially available Blood Glucose Meter (LifeScan, Malvern, PA). An enzyme-linked immunosorbent assay (ELISA) kit (KE20008, Proteintech, Wuhan, China) was employed to estimate plasma insulin levels as per the manufacturer’s protocols. Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated as HOMA-IR = fasting insulin (mU/l) × fasting blood glucose (mmol/l)/22.5.
Morris water maze test
Twenty-four hours after the last drug administration, rat spatial learning and memory were estimated using the Morris water maze (MWM) test according to the previous description [9]. The equipment consisted of a circular pool (120 cm diameter; 50 cm height) filled with opaque water and a platform (10 cm diameter) placed in the middle of the target quadrant under 1.5 cm of water. Animal behaviors were video-recorded and analyzed by an automated analysis system (Daheng Group, Beijing, China). In training trials (days 1-5), rats were gently placed in the pool and allowed to swim freely to the hidden platform within 60 s (four times a day, with a 2-h interval). The time that the rat spent in finding the platform was recorded as the escape latency. If the animal failed to locate the platform within 60 s, it was placed on the platform for 10 s, and the escape latency was recorded as 60 s. On day 6 (24 h after the last training trial), the hidden platform was removed; rats were placed in the pool and allowed to swim freely for 60 s. Each rat was tested only once in the probe trial. The time that rats spent in the target quadrant was recorded.
Sample collection
After the MWM test, the rats were euthanized under anesthesia by cervical dislocation. The brain was exposed, and hippocampal tissues were excised and washed with normal saline to remove residual blood. The hippocampal tissues from a portion of the rats (n = 6) were fixed in 10% buffered formalin for histologic observation, and those tissues from the remaining 6 mice were stored at –80oC for subsequent analysis.
Hematoxylin-eosin (H&E) staining
The hippocampal tissues were dehydrated, paraffin-embedded, and sectioned (5-µm thickness). The sections were then dewaxed and rehydrated, followed by staining with H&E (Solarbio, Beijing, China) following the manufacturer’s protocols. The results were observed using a microscope (Leica Microsystems, Shanghai, China). A relatively constant number of pyramidal neurons from each visual field was photographed. The number of cells in the same area in each photo was counted, and the average of three sections was taken as the number of surviving neurons in the tissue sample.
Immunofluorescence (IF) staining
Rat hippocampal tissue sections were deparaffined and rehydrated, followed by heat-mediated antigen retrieval using EDTA buffer (pH 8.0). Next, the sections were incubated with anti-p-tau antibody (ab92676, 1 : 50, Abcam, Shanghai, China) at 4oC overnight, followed by incubation with Goat Anti-Rabbit IgG H&L (Alexa Fluor 488) secondary antibody (ab150077, 1 : 200) at room temperature for 1 h. DAPI (Solarbio) was employed for nuclear labeling. After being mounted and sealed, tissues were observed under a fluorescence microscope (Leica Microsystems). Signal intensity was quantified using ImageJ software.
ELISA
The hippocampal tissues were homogenized and centrifuged at 4oC for 15 min at 3500 rpm. The resulting supernatant was collected to estimate the concentrations of inflammatory mediators, including TNF-α (CSB-E11987r), IL-1β (CSB-E08055r), IL-6 (CSB-E04640r), and NF-κB (CSB-E08788r) using corresponding rat ELISA kits (CUSABIO, Wuhan, China) as per the manufacturer’s recommendations.
Western blotting
Protein isolation from rat hippocampus was achieved using RIPA buffer (Beyotime), followed by quantification of protein concentration using a bicinchoninic acid assay kit (Beyotime). Protein samples (20 µg) were resolved in 10% SDS-PAGE, blotted on polyvinylidene difluoride membranes (Beyotime), and then blocked with 5% defatted milk. Afterward, the membranes were incubated at 4oC overnight with primary antibodies against p-Akt (ab38449, 1 : 500), Akt (ab8805, 1 : 500), p-PI3K (ab182651, 1 : 200), PI3K (ab191606, 1 : 1000), p-GSK3β (ab227208, 1 : 500) and GSK-3β (ab107166, 1 µg/ml) (all from Abcam). The secondary antibody (ab97080, 1 : 5000, Abcam) was used for subsequent incubation at room temperature for 2 h. Lastly, blot signals were detected using BeyoECL plus (Beyotime) and analyzed using ImageJ software (NIH, Bethesda, CA).
Statistical analysis
Data are presented as the mean ± standard deviation. Difference comparisons among groups were estimated using one-way ANOVA using GraphPad Prism software (GraphPad, San Diego, CA). Two-way ANOVA was used for escape latency analysis in the MWM test, blood glucose, and insulin level analysis. Tukey’s post hoc test was used for all analyses. P < 0.05 indicated statistical significance.
Results
Swertiamarin improves cognitive function in T2D rats
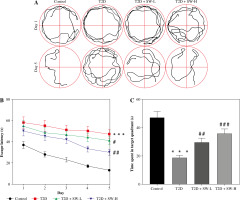
The MWM test was conducted to estimate the cognitive function of rats in each group. As shown in Figure 2A, after 5 days of training, the swimming trajectory of T2D rats was much longer and more chaotic than that of the control rats, while this situation was improved in SW-treated groups, especially in the SW-L group. Figure 2B shows that T2D rats had longer escape latency than the control rats, whereas SW administration, especially SW-H, reduced the escape latency of T2D rats, indicating that SW alleviated diabetes-evoked impairment of spatial learning in rats. In parallel, the results of the probe test on day 6 indicated that relative to the control rats, T2D rats spent less time in the target quadrant (Fig. 2C), suggesting the impaired memory of T2D rats. However, the time that T2D rats spent in the target quadrant was markedly increased after SW administration, especially SW-H (Fig. 2C). Collectively, these results revealed that SW could ameliorate cognitive deficits in T2D rats.
Fig. 2
Swertiamarin (SW) improves cognitive function in type 2 diabetes (T2D) rats. A) Swimming trajectory of rats in each group on days 1 and 5 of training. B) Escape latency on days 1-5 of training. C) Measurement of the time spent in the target quadrant on day 6. ***p < 0.001 vs. control group, #p < 0.05, ##p < 0.01, ###p < 0.001 vs. T2D group
Swertiamarin relieves hyperglycemia and insulin resistance in T2D rats
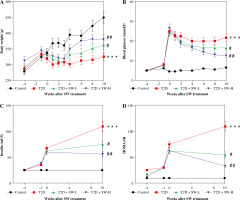
As shown in Figure 3A, STZ injection induced weight loss in T2D rats. However, the body weight of T2D rats continued to grow after the administration of SW, particularly SW-H (Fig. 3A). We estimated the blood glucose and insulin levels of rats. As the results showed, STZ injection dramatically elevated fasting blood glucose levels in rats, whereas SW treatment dose-dependently reduced blood glucose levels in T2D rats (Fig. 3B). In parallel, STZ injection enhanced plasma insulin level and HOMA-IR (Fig. 3C, D), confirming that the T2D model was successfully induced by HF diet feeding plus STZ injection. However, in T2D rats, SW administration remarkably reduced plasma insulin levels and HOMA-IR, with SW-H showing a more pronounced effect (Fig. 3C,D). These data suggested that SW could ameliorate hyperglycemia and insulin resistance in T2D rats.
Fig. 3
Swertiamarin (SW) relieves hyperglycemia and insulin resistance in type 2 diabetes (T2D) rats. A) Body weight of rats in each group. B) Fasting blood glucose level in each group. C) Fasting plasma insulin level. D) Measurement of HOMA-IR. ***p < 0.001 vs. control group, #p < 0.05, ##p < 0.01 vs. T2D group
Swertiamarin ameliorates hippocampal pathological changes and tau hyperphosphorylation in T2D rats
H&E staining was performed to examine whether SW affected hippocampal morphology in rats. The control group showed normal hippocampal morphology, with clear nuclear membranes and distinct nucleoli, while the T2D group exhibited significant loss of hippocampal neurons and sparse arrangement of hippocampal cells with blurred boundaries. However, these abnormalities were markedly abated after SW administration (Fig. 4A). Consistently, semi-quantitative analysis showed that the T2D + SW groups, especially the T2D + SW-H group, had more hippocampal neurons than the T2D group (Fig. 4C). Moreover, significant tau hyperphosphorylation was observed in the hippocampus of T2D rats in comparison to the control rats, whereas SW administration markedly reduced p-tau expression in T2D rat hippocampus, as shown by IF staining (Fig. 4B, 4D).
Fig. 4
Swertiamarin (SW) ameliorates hippocampal pathological changes and tau hyperphosphorylation in type 2 diabetes (T2D) rats. A) Representative images of H&E staining for morphological observation of rat hippocampus in each group. B) Representative images of IF staining for detecting p-tau expression in rat hippocampus. C) Semi-quantitative analysis of the number of hippocampal neurons in each group. D) Quantitative results of the fluorescence intensity of p-tau. ***p < 0.001 vs. control group, ##p < 0.01, ###p < 0.001 vs. T2D group
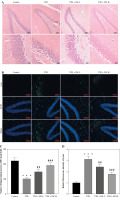
Swertiamarin reduces inflammatory mediator production T2D rat hippocampal tissues
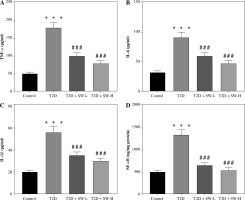
Subsequently, we estimated the SW effect on the production of inflammatory mediators in rat brain tissues. As expected, the levels of proinflammatory mediators, including TNF-α, IL-6, IL-1β, and NF-κB, in the T2D group were prominently higher than those in the control group (Fig. 5A-D), indicating that diabetes led to neuroinflammation in rats. Of note, SW treatment markedly counteracted the above effects in T2D rats (Fig. 5A-D), suggesting the anti-inflammatory effect on the hippocampus of T2D rats.
Swertiamarin activates PI3K/Akt/GSK3β signaling in the hippocampus of T2D rats
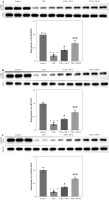
To explore the potential mechanism by which SW affected the progression of DE, we estimated its effect on PI3K/Akt/GSK3β signaling in the hippocampus of T2D rats. As depicted by western blotting, relative to the control rats, T2D rats showed markedly lower levels of p-PI3K, p-Akt, and p-GSK3β in the hippocampal tissues (Fig. 6A-C). However, the above effects were prominently reversed by SW treatment, especially SW-H, in T2D rats (Fig. 6A-C), indicating that SW restored diabetes-triggered inactivation of PI3K/Akt/GSK3β signaling in rat hippocampus.
Fig. 6
Swertiamarin (SW) activates PI3K/Akt/GSK3β signaling type 2 diabetes rat hippocampal tissues. A, B) Western blotting for detecting protein levels of p-PI3K and p-Akt in the rat hippocampus of each group. ***p < 0.001 vs. control group, #p < 0.05, ###p < 0.001 vs. T2D group. Swertiamarin (SW) activates PI3K/Akt/GSK3β signaling type 2 diabetes rat hippocampal tissues. C) Western blotting for detecting protein levels of p-GSK3β in the rat hippocampus of each group. ***p < 0.001 vs. control group, #p < 0.05, ###p < 0.001 vs. T2D group
Discussion
The present study demonstrated that SW treatment could improve the spatial learning and memory of T2D rats and ameliorate hyperglycemia as well as insulin resistance in T2D rats. In addition, we found that SW could alleviate hippocampal morphological changes, reduce tau hyperphosphorylation, and attenuate neuroinflammation in the hippocampal tissues of T2D rats. Moreover, SW activated PI3K/Akt/GSK3β signaling transduction in rat hippocampus, which might be the key mechanism of the neuroprotective function of SW.
Type 2 diabetes is characterized by insulin resistance that causes glucose intolerance and hyperglycemia [20]. Mounting evidence has suggested that prolonged hyperglycemia is associated with morphological and functional changes in the central nervous system [21]. Chronic hyperglycemia induces persistent glucotoxicity in the brain and leads to severe neuropathologic changes such as neurodegeneration, neuroinflammation, and cognitive dysfunction [7]. STZ is a glucosamine derivative of nitrosourea and has been widely used in T2D induction in animal models. Here, we established a rat T2D model by HF diet feeding and STZ injection. Consistent with previous evidence, our results showed that T2D rats exhibited impaired spatial learning and memory abilities. However, SW treatment dose-dependently improved T2D-induced cognitive dysfunction in rats. Moreover, previous reports have indicated that SW possesses anti-hyperglycemic activity in STZ-induced T2D rats [11]. Moreover, Xu et al. proposed the ameliorative effect of SW on glucose intolerance and insulin resistance in HF diet-fed mice [12]. Similarly, in this study, SW administration induced a significant reduction in levels of fasting blood glucose and insulin in T2D rats, confirming its beneficial effects on glucose metabolism and insulin resistance in rats with T2D.
Mounting evidence has revealed that neuroinflammation is a crucial driver of DE progression [22]. Elevated production of proinflammatory cytokines is closely linked to hippocampal neuron injury and cognitive deficits in T2D [23]. SW has been reported to have potent anti-inflammatory properties in several inflammatory disorders [24, 25]. In this study, we found that SW markedly counteracted T2D-triggered increases in proinflammatory mediator levels in the rat hippocampus. Of note, other studies showed that SW could reduce the levels of several proinflammatory factors, including IL-1β, IL-6, and TNF-α, in lipopolysaccharide-stimulated neurons [13], which partially supports our results in this study.
Tau is a neuronal cytoskeletal protein that facilitates the assembly and stability of microtubules [26]. Tau hyperphosphorylation contributes to cognitive dysfunction in DE [27]. Evidence suggests that high glucose can trigger tau phosphorylation in hippocampal neurons, and hyperphosphorylated tau has been observed in the brains of diabetic patients and experimental animals [28]. Consistently, our results revealed a marked increase in p-tau levels in the hippocampus of T2D rats. Furthermore, to our knowledge, our study indicated for the first time that SW administration could hinder hyperglycemia-induced tau hyperphosphorylation in T2D rat hippocampal tissues, indicating the neuroprotective effect of SW on T2D-induced DE.
Previous research has demonstrated that interfering with insulin signaling pathways, such as PI3K/Akt signaling, results in insulin resistance, consequently leading to T2D and the associated complications [29]. GSK3β is a downstream substrate of PI3K/Akt signaling and phosphorylation at Ser9 by Akt is essential to repress its activity [30]. Activated GSK-3β aggravates neuroinflammation and tau phosphorylation [8]. GSK3β phosphorylation at the Ser9 site has been previously detected in postmortem AD brain specimens [30]. Activation of the PI3K/Akt/GSK3β pathway in the hippocampus protects against T2D-induced DE [31]. In accordance with previous reports, we observed that T2D rats exhibited decreased p-PI3K, p-Akt, and p-GSK3β in the hippocampus. Nevertheless, SW treatment restored the phosphorylation of PI3K, Akt, and GSK3β in T2D rat hippocampal tissues, indicating that the neuroprotective effect of SW on T2D rats might be associated with activation of PI3K/Akt/GSK3β signaling in the brain.
In conclusion, this study revealed that SW has a neuroprotective function in T2D rats by attenuating hyperglycemia, tau hyperphosphorylation, and neuroinflammation possibly via activation of the PI3K/Akt/GSK3β pathway. Our findings may provide a new clue for treating T2D-induced DE. Additionally, future studies are required to elucidate the mechanism underlying the beneficial effects of SW.