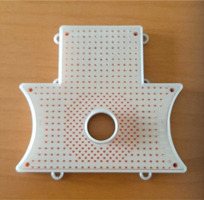
Purpose
The development of precise and adaptable applicators in interventional radiotherapy (IRT, brachytherapy [BT]) for perineal malignancies, especially for anal canal cancer, presents significant challenges due to the complex anatomy and specific requirements involved. In fact, internal pelvic organ conformational changes and alterations in spatial relationships between these organs [1, 2] can impact procedural accuracy and outcomes [3, 4]. Moreover, procedures often require a tailored template configuration to ensure accuracy, which is difficult to achieve with a one-size-fits-all approach.
Furthermore, the need for secure anchoring to immobilization systems, such as stepper devices, adds another layer of complexity to the design of these applicators. Our report introduces a new applicator design, which directly addresses these issues by providing a versatile and adaptable solution that minimizes organ conformation, maintains correct anatomical relationships, and can be customized for various perineal malignancies.
Numerous studies have been conducted to compare external beam boost technique with interventional radiotherapy [5-8]. The use of this type of treatment demonstrates a more acceptable toxicity profile, with a reduction in side effects, such as proctitis, providing encouraging results in terms of local control of the disease.
In 1971, Papillon et al. developed a new approach known as the Lyon technique [9]. The use of radium was replaced by iridium-192 (192Ir) wires, and the interventional radiotherapy method was further optimized in line with the advancements in both understanding of the disease’s progression and technological innovations. A 15 mm thick plastic template, either crescent-shaped or circular, with guiding holes was stitched to the skin. The implanted area covered the same quadrant and depth as the original tumor extension.
Papillon’s protocol has been developed as part of a broader and progressive European experience with interventional radiotherapy [10-12]. In most studies, different doses were administered using either low-dose-rate (LDR) or pulsed-dose-rate (PDR) techniques, while emerging evidence suggests growing experience with high-dose-rate (HDR) interventional radiotherapy [13-16]. Particularly in anal cancer, HDR interventional radiotherapy, often administered as a boost following external beam radiotherapy (EBRT) and chemotherapy, was shown to enhance local control while limiting toxicity, especially when guided by transrectal ultrasound (TRUS). Nevertheless, to preserve sphincter function and minimize the risk of incontinence or necrosis, specific anatomical and technical limitations must be considered. The implantation should not exceed 50% of the anal circumference, and both the longitudinal extension and thickness of the tumor should guide needle placement and dose distribution. The adoption of imaging-guided techniques, such as TRUS, has contributed to reducing complications and improving the precision of dose delivery [17, 18].
The current study presented an innovative “all-in-one” applicator designed to address challenges in IRT for tumors treatable trough a perineal implant (i.e., the vulva, vagina, anal canal, and prostate) using various types of diagnostic imaging (i.e., MRI, CT, PET/CT, and US). The invention is called the TIMER® applicator (gemelli arT Interstitial Multi-imaging pERineal applicator). The acronym suggests also a special tool, which could be used in a time-sparing procedure due to its versatility.
The aim of this article was to provide a dosimetric comparison between the TIMER® applicator and two other applicators already in clinical use. This comparison aimed to assess the effectiveness of the new applicator in terms of dose distribution, precision, and adaptability, highlighting its potential advantages in clinical practice with a special focus on anal canal cancer.
Material and methods
The TIMER® applicator proposed in this article, illustrated in Figure 1, has been developed with the aim of advancing areas dose distributions of perineal tumors by IRT.
MRI/CT-compatible material
For prostate, anal, vaginal, and vulvar tumors, MRI [19] offers advantages over ultrasound (US) in identifying disease. The applicator is entirely made of plastic materials without metallic parts. This feature allows safe MRI and CT imaging, preventing artifacts’ interference with diagnostic image analysis. The applicator can be used both for direct IRT in a MRI room and for pre-planning in MRI simulation, with subsequent image fusion with CT or US before and/or after implantation. Using the same applicator in both phases of the therapeutic process ensure the same shape of organs and stable anatomical positioning of the patient, thereby enhancing the accuracy of image fusion.
Dual anchoring options
The applicator can be secured directly to the patient’s skin using sutures through rings placed externally on the template or attached to a stepper and immobilizer for ultrasound guidance. The first configuration is useful for treatments of the vulva, vagina, and anus, while the second can be used for treatments of the prostate, anterior anus, anterior vulva, and anterior vagina. The geometry of the needle holes aligns with the anchor screw holes for the ultrasound mount, allowing needle insertion even in positions designated for anchoring screws.
Echo-transparent central cylinder
The applicator has an echo-transparent central cylinder useful for both implant stabilization and ultrasound-guided procedure. Performing an implant in a MRI room requires complex processes [20], leading to a significant use of operator time and occupation of the MRI room, limiting availability for other activities. One proposed solution is image fusion between a previously acquired MRI and an ultrasound obtained during interventional procedure. However, the accuracy of this process can be complicated by anatomical differences between the two stages, as inserting an ultrasound probe into the anus/rectum or vagina dilates these organs, thus altering their shape and displacing adjacent organs, such as the prostate. Using the applicator during both MRI and ultrasound imaging allows image acquisition without local anatomical modifications, achieving high precision in aligning the two imaging modalities. This process is useful for both therapeutic interventions (radiotherapy, electrochemotherapy, etc.) and diagnostic procedures (i.e., biopsies). An ultrasound-compatible sleeve was tested using a phantom to verify imaging performance. The material did not introduce any artifacts or impair image quality, confirming its suitability for ultrasound-guided procedures.
Inverted T-shape with lateral concave form
This unique shape allows the applicator to be used for procedures involving the anus, vagina, vulva, or prostate due to its vertical projection. Additionally, the lateral concave form provides better anatomical adaptation to patients in the lithotomy position, following the natural contour of thigh’s origin.
Pressure needle locking system
This system is similar to those found in other commercial applicators, but differs in MRI compatibility, as the device is MR-safe. The applicator is composed of two rigid plastic parts, and a flexible, rubber-like part. At the end of the implant, by tightening the plastic screws, the rigid parts compress the flexible part, securing the needles and preventing longitudinal movement.
Needle insertion hole distribution
The hole pattern is evenly distributed across the applicator’s surface, except around the cylinder where hole density increases. The grid layout allows easy localization of holes during implantation. A higher density around the cylinder enables a greater number of needles to be used in the area surrounding the urethra for vulvar and vaginal treatments or around the anus for anal treatments. Also, a higher needle density allows better dose modulation, thereby reducing side effects associated with the treatment.
Clinical advantage lies not in inserting a higher number of needles, but in having a dense array of potential positions to selectively optimize the implant geometry. Several studies have shown that increasing the number of actual needle insertions may elevate the risk of acute genitourinary and gastrointestinal toxicity, particularly when needles are repeatedly manipulated or placed near sensitive structures [21, 22]. Therefore, the ability to choose the most appropriate positions from a wide configuration supports both dose distribution and toxicity reduction.
From a planning perspective, access to a high-resolution spatial grid allows flexibility and adaptability during treatment. Evidence suggests that treatment quality improves when needle positioning is tailored to anatomy and dose constraints, rather than maximized indiscriminately [23, 24].
Dosimetric aspects
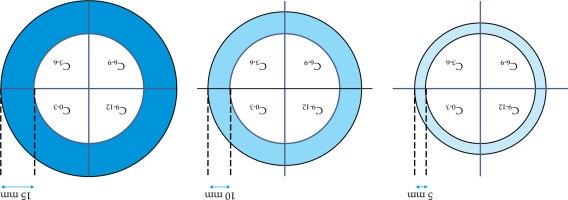
A dosimetric evaluation was conducted by comparing two applicators (Applicator 1 and Applicator 2), shown in Figure 2, which are already commercially available and used in clinical practice, with the TIMER® applicator developed in our center for interstitial IRT. For each template, a CT scan of these 3 applicators was performed in order to establish the configuration of holes within each template, followed by a treatment plan using TPS Oncentra Brachy (Elekta®). A forward planning approach was employed to standardize catheter activation and allow consistent comparison among templates. Dwell positions were defined every 2 mm along each catheter to increase spatial resolution in the dose modelling process. Although this differs from the typical 5 mm spacing adopted in clinical settings due to source length, the 2 mm interval was chosen to enhance accuracy in dosimetric evaluation. Additionally, we created different clinical target volume (CTV) configurations at varying thicknesses from the anal canal, as demonstrated in Figure 3, to simulate a range of possible target volumes. In particular, in the cross configuration shown, the entire circumference was divided into four zones (from 0 to 3 o’clock, from 3 to 6, from 6 to 9, and from 9 to 12), creating a cross pattern. For each zone, specific CTVs were established at different thicknesses, such as 5 mm, 10 mm, and 15 mm, to test dose distribution across different target sizes and shapes, capturing clinical variations.
Fig. 2
Illustration of the needle distribution for the commercially available applicators referred to in this article as Applicator 1 and Applicator 2
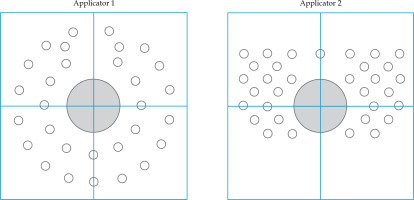
Fig. 3
In cross configuration, the circumference is divided into four zones at right angles. Each zone includes specific CTVs with thicknesses of 5 mm, 10 mm, and 15 mm
To assess which template performed best given an equivalent target volume coverage (i.e., 95% of the prescription dose to 95% of the volume), we considered dose non-homogeneity ratio (DNR [25]). The DNR, defined as the ratio between the volumes receiving 100% and 150% of the prescription dose, provides an indication of dose homogeneity within the target volume. A lower DNR value reflects a reduced high-dose region within the target, and indicates a more uniform dose distribution, which is favorable for minimizing over-irradiation.
Following the CTV configuration, needles were reconstructed for each device according to a standardized approach: all interstitial needles located outside the largest CTV were limited to a maximum distance of 5 mm from the CTV margin, with placement based on the Paris system. During the reconstruction, needles positioned within the mucosal region were excluded, as these could contribute to excessive high-dose delivery to the adjacent mucosa. Therefore, an interstitial only approach was selected. Needles configurations varied depending on the template used and the unique hole arrangement of each template.
An automated activation process was utilized for each CTV, with a 5 mm margin to activate needles in close proximity for optimal dose coverage. Dose normalization was subsequently done to achieve the exact coverage of 95% of the 95% of target volume. Activated points were defined at 2 mm intervals along each catheter, following consistent spacing to maintain reproducibility across the templates.
This methodology was used to ensure a comprehensive analysis of dose coverage across a realistic spectrum of target volumes, adjusted as much as possible according to each template’s structure and configuration.
No inferential statistical tests were applied, given the exploratory and technical nature of the analysis. The study aimed to identify trends and patterns for clinical considerations rather than to test hypotheses.
Results
The analysis of DNR values is displayed along with the heatmap in Figure 4, where higher values indicate poorer performance according to the metric definition. This heatmap allows to evaluate device performance (Applicator 1, Applicator 2, and TIMER®) across various depths and configurations. In particular, in Figure 4, DNR values for each device are presented at depths of 5 mm, 10 mm, and 15 mm, with configurations labelled as C0-3, C3-6, C6-9, and C9-12. The spectral color palette allows differentiation between higher and lower DNR values, thus demonstrating that the optimal applicator varies depending on CTV configuration.
Fig. 4
Heatmap displaying the distribution of non-uniformity ratio (DNR) values across different depths and configurations for three applicators: APP. 1, APP. 2, and TIMER®
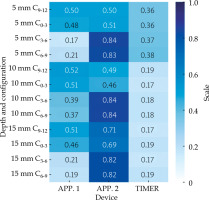
In general, the TIMER® applicator consistently outperformed the others, except for the case of a shallow posterior CTV (5 mm), where APP. 1 exhibited slightly better performance (DNRAPP1 = 0.17 vs. DNRTIMER = 0.37 for configuration C3-6, and DNRAPP1 = 0.21 vs. DNR-TIMER = 0.38 for configuration C6-9). However, even in this configuration, TIMER remained superior to APP. 2 (DNRAPP2 > 0.8 for configurations C3-6 and C6-9).
APP. 1 generally performed better than APP. 2, especially for posterior CTV configurations and at shallower depths of 5 mm. Conversely, APP. 2 showed the lowest performance overall, with one notable exception, i.e., for an anterior CTV at 10 mm, APP. 2 performed (slightly) better than APP. 1 (DNRAPP1 = 0.52 vs. DNRAPP2 = 0.49 for configuration C9-12, and DNRAPP1 = 0.51 vs. DNRAPP2 = 0.46 for configuration C0-3), yet it still did not surpass the TIMER® applicator.
The heatmap further reveals that TIMER maintained lower DNR values across nearly all configurations, highlighting its robustness as CTV depth increases. To complement the quantitative analysis, representative examples of needle configurations for selected CTV scenarios are provided in Figure 5. These cases illustrate how unique geometry of each applicator influenced needle placement and, consequently, dose distribution.
Fig. 5
Representative examples of needle configurations for CTV (10 mm 3-6) with different applicators: APP. 1 (A), APP. 2 (B), and TIMER® (C)
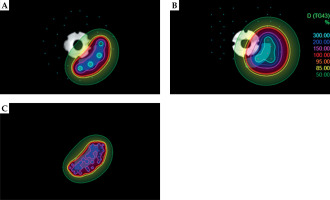
In summary, TIMER® emerges as the most versatile applicator, consistently performing well across different configurations, while APP. 1 serves as a feasible alternative at shallow depths, especially for posterior CTVs. APP. 2, on the other hand, has limited applicability, with specific potential only at intermediate CTV depths in anterior regions. This comprehensive analysis underscored TIMER®’s superior performance, the potential of APP. 1 for shallow CTVs, and the restricted utility of APP. 2 across the configurations examined.
Discussion
The dosimetric analysis presented in this paper provides a detailed evaluation of dose distribution capabilities among different applicators used in interstitial IRT of the anal canal. By examining distinct CTV configurations at varying thicknesses as well as assessing dose distribution through coverage and DNR, this study offers valuable insights into each device’s suitability for specific clinical application.
The results demonstrate that TIMER® consistently outperforms the other applicators by maintaining lower DNR values across various depths and configurations due to its well-distributed catheter positioning. This feature makes TIMER® highly effective across a range of tissue depths and treatment areas, especially where dose uniformity is desired. Applicator 1, while generally adaptable with variable DNR values across depths and configurations, showed a clear advantage over TIMER® only for posterior lesions at a shallow depth of 5 mm. In most other configurations, TIMER®’s superior catheter distribution provided a more reliable and homogeneous dose coverage.
Applicator 2, by contrast, tended to demonstrate higher DNR values, particularly at greater depths, suggesting that its dose distribution is less uniform and potentially less effective in deeper tissue applications. However, it should be noted that for posterior lesions, Applicator 2 could be rotated 180 degrees to position the available catheters lower. As a result, the DNR values observed for configurations C9-12 and C0-3 could also apply to the C3-6 and C6-9 zones. In this way, DNR would significantly improve and become comparable to that of Applicator 1, although still inferior to TIMER®.
While the catheter arrangement in both Applicator 1 and Applicator 2 creates some variability in DNR across sectors, TIMER®’s more consistent DNR performance highlights its robustness. Even though TIMER® showed strong performance, one limitation in this study is that the prototype’s current catheter positions may not fully cover very thin lesions. Future implementations of TIMER® could enhance dose coverage by positioning additional catheters in closer proximity to improve dose distribution for thin target volumes.
In summary, this dosimetric analysis highlights TIMER® as the superior choice among the tested applicators, especially due to its consistency across positions and depths. Applicator 1, with some adaptability, might be suitable in specific shallow or posterior applications, while Applicator 2 appears less suitable for scenarios requiring dose uniformity. These findings underscore the importance of choosing IRT devices based on dose distribution requirements, and validate DNR as an essential metric for guiding clinical decisions. Future studies should focus on linking these dosimetric patterns to clinical outcomes, refining device selection criteria for IRT applications.
Conclusions
The TIMER® applicator significantly improves IRT for perineal malignancies by addressing challenges in anatomy and treatment precision. Its MRI/CT compatibility, adaptable anchoring, and optimized needle layout, ensure superior dose uniformity and procedural accuracy compared with commercially available templates. Its versatility across various clinical scenarios underscores its potential to enhance outcomes and minimize toxicity. Future refinements and clinical validation will solidify its role in advancing IRT protocols.