Purpose
Chest wall cancer (CWC) may derive from a variety of cancers, including breast cancer, mesothelioma, sarcoma, lung cancer, and esophageal cancer [1-3]. Recurrent CWC (rCWC) is complicated with limited salvage therapy after failure of surgery/external beam radiotherapy (EBRT) [3-5]. Chemotherapy with or without local therapy is a palliative option. Salvage surgical resection/EBRT may be used in selected patients to improve local control depending on the number and extensions of the lesions. The extent of resection significantly influences survival, with satisfying clinical outcome absent in many patients who can only receive limited resection, because extensive resection carry a high-risk of disfiguring [3]. EBRT is an alternative option for patients without prior EBRT; however, it is hard to deliver a high-dose to the lesions owing to the movement and irregular nature of rCWC. It is difficult to use for patients with prior EBRT with a high-risk of radiation-related toxicities, such as chest fibrosis, pneumonia, and rib fracture [5].
Computed tomography (CT)-guided iodine-125 seed implantation (125I-SI) is proved to be safe and effective when applied in head and neck, thoracic, abdomen, retroperitoneal, and vertebral cancers, especially for locally advanced or recurrent solid cancers after EBRT [6-12]. 125I-SI may be applied as a salvage therapy for rCWC alone or as a boost to EBRT, due to rapid dose falling-off and sparing of surrounding organs, while the chest wall is irregular and it may be difficult to perform CT-guided 125I-SI in patients with rCWC. Organ motion and non-uniformity of the organ may interference with the needle insertion during seed implantation and lead to a sub-optimal distribution of seeds, partly due to the ribs interference [10].
The main challenge is how to deliver the 125I seeds into the target volume accurately according to pre-plan. 3D-printing non-co-planar template (3D-PNCT) is helpful for high quality performance of CT-guided 125I-SI [7, 8, 12-14]. The safety, accuracy, and feasibility of 3D-PNCT application in CT-guided 125I-SI for cancers raised in complex locations, such as the head and neck, has been explored and reported [7, 8, 10, 12-15]. However, the literature on the accuracy and dosimetric analysis of 3D-PNCT-assisted 125I-SI for rCWC is scarce. Therefore, the present retrospective study analyzed the accuracy and dosimetric parameters of 3D-PNCT-assisted CT-guided 125I-SI in patients with rCWC.
Material and methods
Study design and population
In the retrospective study, 19 consecutive patients with 22 rCWC lesions treated with 125I-SI between Mar 2017 and Mar 2020 were included. Among them, 14 patients were previously treated with EBRT once, 2 patients were treated twice, and the remaining 3 patients refused EBRT (Table 1). The inclusion criteria of 125I-SI were as follows: 1) age 18-80 years, Karnofsky performance status (KPS) score more than 70; 2) pathologically or radiologically diagnosed rCWC (defined as primary/secondary cancers raised from the chest/cervicothoracic junction); 3) the diameter of the lesion less than 7 cm, the number of lesions less than 3 [8]; 4) expected survival time more than 3 months; and 5) intolerant/refused to surgery/EBRT. Contraindications were as follows: 1) severe organ function disorder; 2) coagulation dysfunction; 3) recent active infection; 4) mental illness; and 5) lesions invasive to the skin (avoiding refractory skin ulcer that may be caused by 125I-SI).
Table 1
Characteristics of the 19 patients
The number of needles and seeds, deviation of needles entrance, angle and depth for pre-plan, and the intra-operative real-time plan were measured on brachytherapy treatment planning system (B-TPS) according to a previous report [13]. The dosimetric parameters including D90, D100, V100, V150, V200, conformity index (CI), external index (EI), and homogeneity index (HI) were compared between pre-plan and post-plan. The dosimetric parameters were defined as doses delivered to 90% (D90) and 100% (D100) of gross tumor volume (GTV) and percentage of GTV receiving 100% (V100), 150% (V150), and 200% (V200) of the prescription dose. The peri-operative adverse events were evaluated during 1 month after 125I-SI according to the Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC) criteria [16]. The cancer response and patient survival were recorded. Pain was routinely assessed using a numerical rating scale (NRS), which was categorized into five grades: 0 for no pain, 1-3 for mild pain, 4-6 for moderate pain, 7-9 for severe pain, and 10 for unbearable pain [17]. The NRS score before 125I-SI was compared with that of 1 month after 125I-SI.
Pre-plan
The patients were immobilized with a vacuum pad in a suitable position and underwent an enhanced CT (Brilliance, Philips Inc., The Netherlands) scan with a 5 mm slice thickness 1-2 days before 125I-SI. The position lines were marked on the patient skin according to the tumor’s central location. CT simulation images were transferred into B-TPS (Beijing Aeronautics and Astronautics University and Beijing Astro Technology Co., Ltd.). Both GTV and organs at risks (OARs) were defined and delineated. Clinical tumor volume (CTV) was defined by expanding 5-6 mm in 3D directions from GTV. The median prescription dose was 120 Gy (range, 110-160 Gy), and the median activity of 125I seed (the length 4.5 mm, diameter 0.8 mm, and half-life 59.4 days; Beijing Atom High Tech Pharmaceutical Company Inc., China) was 0.55 mCi (range, 0.40-0.75 mCi). Dosimetric optimization was conducted and the D90 was optimized as close as to the prescription dose, whereas the doses of OARs were kept as low as possible.
3D-PNCT design and printing
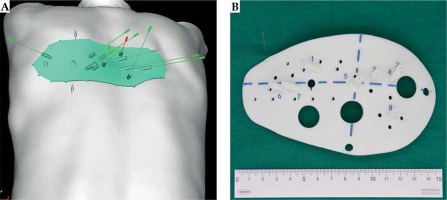
The number of needles and seeds, needles pathway, and seeds distribution were designed and simulated on B-TPS. The dose conformity of CTV was optimized while sparing doses to surrounding normal tissues. OARs, such as blood vessels, bony structure, and nerves, were avoided and kept away from the needle pathway usually with a safety distance of 0.5 cm. The data was imported to Magics 19.01 (Materialise, Belgium) for digital modeling of individualized 3D-PNCT (Figure 1A). Then, 3D-PNCT was printed using a 3D light-cured rapid-forming printer (RS6000, Shanghai Liantaiv 3D Technology Company Inc., China). The 3D-PNCT consisted of anatomical information of the target area, needle pathway and entrance, and position lines, etc. (Figure 1B). Additional 2-3 holes for anchoring needles were added to fix the 3D-PNCT to the targeted area of the patients’ body.
125I-SI procedure
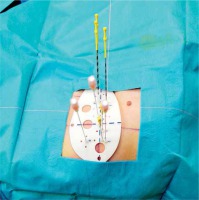
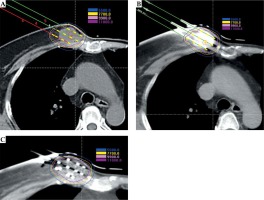
The 125I-SI procedure has been described in our previous published literature [11]. In brief, the procedure was as follows: 1) Patients were immobilized with a vacuum pad. Local anesthesia was carried out. The 3D-PNCT was aligned to the target area of the patients’ bodies according to the laser line marks and position lines (Figure 2). Two or 3 anchoring needles were inserted with 2-3 cm in depth to fix the 3D-PNCT; 2) CT scan was performed to check the anchoring needles position and angles; 3) Adjustment was made when the anchoring needles position was mismatched to pre-plan; 4) The seed needles were then all inserted according to pre-plan, once the anchoring needles were deemed in position; 5) CT scan was performed and adjustment was made when the position of the needle was mismatched to pre-plan, until all the needles were in position. Intra-operative real-time plan was created and conducted when necessary, by adding or dislodging the number of needles/seeds; 6) 125I seeds were delivered and implanted with an applicator (Mick 200-TPV Applicator, Mick Radio-Nuclear Inc., USA) in a regressive manner; 7) CT scan was performed again to verify the actual distribution of 125I seeds, and the images were transferred into B-TPS for a post-plan evaluation. The following data of post-plan were recorded: D90, D100, V100, V150, and V200. All procedures followed the recommendations of the International Commission on Radiological Protection (Figure 3A-C).
Follow-up
The patients were discharged 1-2 days after the 125I-SI procedure; follow-up assessments were routinely performed at 3, 6, 9, and 12 months after 125I-SI and then every 6 months thereafter until disease progression or patients’ death. Follow-up assessments were conducted during regular outpatient visits (9 patients) or telephone interviews (10 patients). CT scans/MRI examinations were used to evaluate the tumor response during each outpatient visit. Modified response evaluation criteria in solid tumors (mRECIST) version 1.1 criteria were used to assess local tumor response in 1 month after 125I-SI, and included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [18].
Statistical analysis
Statistical analysis was performed using SPSS version 25.0 (IBM Corp, Armonk, NY, USA). Paired sample t-test was used to compare mean of pre-operative and post-operative dosimetric parameters. Bland-Altman method was applied to analyze the factors of pre-operative and post-operative dosimetric parameters, using MedCalc v.15.2.2 software. P < 0.05 was considered statistically significant.
Results
The number of needles was 17.19 ±9.32 and 17.29 ±9.22 per patient for pre-plan and post-plan, respectively (p = 0.629). The number of 125I seeds was 74.14 ±43.64 and 79.00 ±48.62 per patient for pre-plan and post-plan, respectively (p = 0.444). The mean needle entrance deviation was 4.50 ±2.70 mm, the mean needle angular deviation was 3.40 ±3.10 degrees, and the mean depth deviation was 5.20 ±5.20 mm.
No significant difference was found for dosimetric parameters (except CI) between pre-plan and post-plan (Table 2). The D90, D100, V100, V150, and V200 were 157.74 ±24.23 and 151.71 ±33.62 (p = 0.228), 85.36 ±34.09 and 70.46 ±23.48 (p = 0.067), 0.93 ±0.04 and 0.90 ±0.07 (p = 0.068), 0.64 ±0.16 and 0.64 ±0.16 (p = 0.984), and 0.35 ±0.17 and 0.37 ±0.18 (p = 0.382) for pre-plan and post-plan, respectively. The CI, EI, and HI were 0.57 ±0.16 and 0.52 ±0.15 (p = 0.007), 0.73 ±0.55 and 0.79 ±0.53 (p = 0.096), and 0.31 ±0.15 and 0.30 ±0.14 (p = 0.504) for pre-plan and post-plan, respectively.
Table 2
Dosimetric parameters of 125I seeds implantation (mean ± SD)
[i] D90/D100 – prescribed dose delivered to 90%/100% of the gross tumor volume, V100 – V150, and V200 – gross tumor volume receiving 100%, 150%, and 200% of the prescribed dose, respectively, CI – conformation index, EI – external volume index, HI – homogeneity index, *p – pre-plan vs. intra-operative, **p – pre-plan vs. post-plan, ***p – intra-operative vs. post-plan
The median follow-up time was 8 months (range, 3-30 months). The CR was observed in 4 of 22 (18.1%), PR in 13 of 22 (59.1%), SD in 4 of 22 (18.1%), and PD in 1 of 22 (4.5%) of the cancers. Among the 8 patients with pain before 125I-SI (5 patients with moderate pain and 3 patients with mild pain), the pain relief rate was 87.5% (7/8). Complete relief was observed after 125I-SI in 2 patients with moderate and 1 patient with mild pain; partial remission was observed after 125I-SI in 3 patients with moderate pain, 1 patient with mild pain (Table 3). Only 1 patient suffered from mild pain during 125I-SI and the pain was immediately relieved after the 125I-SI procedure. No peri-operative complications (e.g., pneumothorax, hematemesis, fever, and infection) of more than grade 2 were observed.
Discussion
Recurrent chest wall cancer tends to invade the skin, rib, or nerves inducing pain, which impairs patients’ quality of life [19-21]. The recurrent lymph node metastasis at the inlet of thoracic invade the tracheal or artery inducing asphyxia and bleeding. The 3D-PNCT is suitable for patients with cancers located in the chest wall or cervicothoracic junction, and the advantages of 125I-SI are as follows: 1) the lesion is relatively superficial and immobilized; 2) the affluent bony structures facilitate 3D-PNCT fixation.
125I-SI as an adjuvant therapy appears to be a feasible modality, and patients recover and return to daily life soon after the treatment due to minimally invasive nature of the 125I-SI procedure. With the CT-guidance, 125I-SI is potentially able to deliver high doses to the target while sparing the surrounding normal tissues, with rapid dose falling-off of accurately implanted seeds. However, the challenge of 125I-SI is how to deliver the radioactive seeds into the targets accurately according to pre-plan in a complex anatomic structures, such as the head and neck, chest wall, retroperitoneal area, and pelvis. The dosimetric parameters of post-plan may not meet pre-plan requirements when the seeds are implanted inaccurately, and the outcomes may be compromised. Wan et al. [22] reported 125I-SI for prostate with an 18-gauge needle to test the accuracy between needle deflection and insertion depth; a needle bending occurred when the needle was inserted deeply. The results showed that the needle tended to diverge and/or bend from its pre-planned trajectory due to asymmetric resistance on the beveled tip of the needle. Rotation of the needle was a solution for decreasing the targeting error. Mean needle targeting errors were 0.8 and 1.2 mm and seeds implanted errors were 0.9 and 1.5 mm for needle rotation and non-needle rotation methods, respectively [22].
The 3D-PNCT was also a useful way to improve the accuracy of 125I-SI obtaining a high-dose in target volume with few needles and complications for advanced or recurrent cancers, particularly for cancers located in complex anatomies [23-25]. Huang et al. [13] reported the accuracy of 3D printing individual templates for 125I-SI in head and neck cancer. Mean needle entrance deviation for all 619 needles was 1.18 ±0.81 mm, varying from 0.857 ±0.545 to 1.930 ±0.843 mm at different sites. Mean needle angular deviation was 2.08 ±1.07 degrees, varying from 1.85 ±0.93 to 2.73 ±1.18 degrees at different sites. Here, the mean needle entrance deviation was 4.50 ±2.70 mm, the mean angular deviation was 3.40 ±3.10 degrees (p = 0.377), and the mean depth deviation of the needles was 5.20 ±5.20. The deviations were all tended to be larger than that reported by Huang et al. in the head and neck, which may partly be due to 1) the breath and chest wall motion during 125I-SI, 2) dense OARs, such as blood vessels, trachea, nerves, etc., 3) 3D-PNCT for the chest was larger than that of the head and neck, which may induce slight 3D-PNCT tortuosity and changes of the needles pathway. The mean post-operative D90 and D100 were 151.71 Gy and 70.46 Gy, respectively. The differences were not significant between the pre-plan and post-plan, which means that the dosimetric data may fulfill the pre-plan requirements during 3D-PNCT-assisted CT-guided 125I-SI. The CI of the post-plan was lower than the pre-plan CI, because seeds position may differ from the pre-plan. Besides, with the assistance of 3D-PNCT during CT-guided interventions, the procedure may be simplified and the operator can be skilled after a relatively short learning time [26].
The present study had several limitations. First, this was a retrospective study with relatively small number of patients, which may lead to potential bias. Second, the absence of control group has also compromised the interpretation of the results. Furthermore, the group of patients was completely heterogeneous concerning tumor sites and pathologies. Finally, the data were obtained from image fusion on B-TPS, which may suffer from potential fusion errors.