Purpose
Sub-total hysterectomy is a prominent surgical procedure for benign uterine diseases that only removes the uterine body and leaves the cervix intact [1]. However, the importance of screening for cervical stump cancer should be emphasized. According to a study with 903 patients in Poland, the prevalence of cervical stump cancer among all cervical cancers is 0.33% [2]. In contrast, another review showed that stump cancer accounted for 2% of all cervical cancers [3]. In developing countries where the human papilloma virus (HPV) vaccine is not widely available, this rate may be higher; however, such data are still lacking.
There is no standard treatment for cervical stump cancer, and the current treatment principles mainly refer to cervical cancer. A study reviewed a series from 1959 to 1987, and found that the effect of radical radiotherapy on cervical stump cancer was similar to that on an intact uterus [4]; however, some studies had shown higher complication rates following radiation treatment for stump cancer [3]. Recently, 3D image-guided brachytherapy (3D-IGBT) was introduced into cervix cancer treatment, resulting in an improved loco-regional control and less toxicity [5, 6]. For example, the EMBRACE I trial showed that, at a median follow-up of 51 months, magnetic resonance imaging (MRI)-guided image-guided adaptive brachytherapy achieved an overall 5-year local control (LC) rate of 92% [7]. However, data on the use of 3D-IGBT for cervical stump cancer are still lacking.
Here, we aimed to summarize the outcomes of cervical stump cancer, and analyze the advantages of 3D brachytherapy in patients’ treatment.
Material and methods
Patients
This retrospective study was approved by the Institutional Review Board of the Peking Union Medical College Hospital (No. JS-2373). Between 2012 and 2021, 33 patients who met the following criteria were initially included in our study: 1) A history of sub-total hysterectomy for benign gynecological diseases; 2) Pathologically proven cervical stump cancer; 3) Received external beam radiation and brachytherapy; 4) Not received other treatment before radiotherapy; and 5) A complete treatment and follow-up data. All patients underwent radiotherapy at the Peking Union Medical College Hospital. After reviewing the data, two participants were excluded due to a loss to follow-up. In addition, demographic features and data associated with treating cancers were collected, including pathological type, FIGO stage, dose of external beam radiotherapy (EBRT), methods and parameters of brachytherapy, and chemotherapy regimen.
For patients treated between 2012 and 2018, stages were re-assessed according to FIGO 2018 and based on their reserved computed tomography (CT) images. Furthermore, information about 2D-BT was described through positions and doses of reference point (point A) and doses of radiation to the bladder, rectum, and fundus of the uterus. In contrast, 3D-IGBT data were described using D90% value of clinical target volume (CTV) and D2cm3 value of the bladder, rectum, small intestine, and sigmoid. Moreover, data on survival and radiation toxicity were collected through telephone follow-ups and periodic re-examinations.
Treatment
All patients were treated with external radiotherapy and brachytherapy. External beam radiation was used to irradiate the cervical, partial vaginal, and pelvic lymphatic drainage areas with volumetric modulated arc therapy (VMAT) or helical tomotherapy (TOMO) at a dose of 45 Gy/25 fractions, or 50.4 Gy/28 fractions. Seven patients received a 10-12 Gy boost for metastatic lymph nodes (Table 1). Twenty-two patients received treatment delivered with Varian Trilogy linear accelerator, with plans generated from Varian Eclipse planning system, while data regarding external radiotherapy units and planning system used for the other 11 patients were missing.
Table 1
Patients’ clinical characteristics
* Doses and locations of these reference points are listed in Appendix Table 1
Tandem and ovoid applicators were applied in all patients undergoing brachytherapy. Thirty-one patients were divided into three groups: 14 patients, who were treated with 2D-BT (2D-BT group) and had their plans based on radiography, and 12 cases, who had preserved CT images at least after the first applicator implantation. Twelve patients treated with 3D-IGBT (3D-IGBT group) had their plans based on CT images, and the other five patients received 2D-BT combined with 3D-IGBT (2D + 3D group) due to incomplete target area coverage in CT simulation assessment after the initial treatment with 2D-BT. Brachytherapy doses were 4-6 Gy per fraction, 2-5 fractions, and were adjusted to achieve a sufficient dose coverage in CTV, while limiting the dose to organs at risk. For 2D-BT, plans based on the images were obtained from oblique orthogonal X-rays. The target area dose was evaluated through point A, and doses to organs at risk (OARs) were represented by markers, such as rectal markers and bladder balloons. Reference points differed among patients because the dose to OARs would be too high if the general point A was applied as the inference point for some patients. Under these conditions, the inference point was adjusted to limit the dose to OARs. For 3D-IGBT, each patient was simulated using an Philips AcQSim CT simulator, with a scanning layer thickness of 5 mm. High-risk clinical target volume (HR-CTV) was delineated based on CT images, including the cervix, adjacent parametrium, and vagina, if involved. A high-dose-rate 192Ir source was adopted; brachytherapy plan was generated using Oncentra brachytherapy treatment planning system (Elekta, Stockholm, Sweden) and was delivered with NucletronV2 with a dwell distance of 2.5 mm for both 2D and 3D brachytherapy. All patients received concurrent chemotherapy.
Dosimetry analysis
To assist in selecting a more individualized reference point, 12 patients in the 2D-BT group obtained CT images at least after the first applicator implantation. We used Elekta Oncentra system to delineate CTV and OARs on these CT images, reconstruct the source applicator, and transplant the dwell position, dwell time, and source activity of the source applicators from the original 2D plans into the simulated 3D-CT plans, thus, assessing their CTV D90% and D2cm3 values in the bladder, rectum, sigmoid colon, and small intestine. For fractions, which missed CT images, we used data received from other fractions of the same patient to replace them. Similarly, these parameters were recorded in 12 patients in the 3D-IGBT group. Biologically equivalent doses in 2 Gy fractions (EQD2) using linear-quadratic model with α/β ratio = 10 for tumors and α/β ratio = 3 for OARs were performed to calculate the superimposed dose of brachytherapy and external beam radiotherapy [8]. Dose target of D90% was set as ≥ 80 Gy, according to the American Brachytherapy Society guideline [9]. Dose constraints of OARs in D2cm3 were set as the bladder < 90 Gy, and the rectum, sigmoid, and bowel < 75 Gy, according to the EMBRACE II study [10].
Follow-up and statistics
After the completion of radiation therapy, patients had their follow-up visits every 3 months within 2 years, every 6 months from 2 to 5 years, and once a year after 5 years. Observations at follow-up included local recurrence, distant metastasis, and death. The prognosis from two aspects, i.e., survival and side effects was evaluated. The survival status was reflected by overall survival (OS), progression-free survival (PFS), and LC rates. OS was defined as the interval between the onset of radiotherapy and death from any cause or the last follow-up; all the death cases were disease-related, so disease-specific survival was referenced to overall survival. PFS and LC were calculated from the start of radiotherapy to any outcome occurrence or local recurrence. Therefore, Kaplan-Meier method was used to estimate OS, PFS, and LC rates. Adverse effects of EBRT and brachytherapy were evaluated based on acute and late toxicities. The most prominent acute and late toxicities of EBRT and BT, including toxicity to the bladder, rectum, sigmoid colon, and small intestine, were assessed according to the common terminology criteria for adverse events v. 3.0 [11].
A relationship between prognostic factors and toxicity was first evaluated using univariate analysis. We defined acute and late toxicities of grade 2 or higher as severe, and analyzed a relationship between each factor and severity of toxicity using chi-square (χ2) test. Variables, which showed significance (p < 0.05) were included in the multivariate model for further analysis. Furthermore, comparisons were made between the 2D-BT and 3D-IGBT groups, while the 2D + 3D group was excluded from the analysis, because it was challenging to determine the source of influence in this group. For dosimetry, the cumulative D90% value of HR-CTV and D2cm3 value of the bladder, rectum, small intestine, and sigmoid colon were compared between the 3D-IGBT and 2D brachytherapy groups using an independent t-test after passing Shapiro-Wilk normality test. P-values < 0.05 were considered statistically significant. All data analyses were performed using SPSS (version 22.0; SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics
General clinical characteristics of the patients are shown in Table 1. The pathological types of 25 (95.7%) patients were squamous cell carcinoma, seven (22.6%) were early-stage (FIGO stages IB1-IIA1), and 24 (77.4%) were locally advanced stage (FIGO stages IIA2-IVA). Among the 14 patients who underwent 2D brachytherapy, only six (42.9%) adopted general point A (at the cervical opening 2 cm upwards and 2 cm from the side). In addition, six types of other reference points were selected (Appendix Table 1).
Prognosis and toxicity
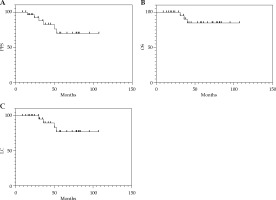
The median follow-up time for the 31 patients was 58 months (range, 16-107 months). The overall 5-year PFS, OS, and LC rates were 69.6%, 84.9%, and 77.1%, respectively (Figure 1). Among them, LC rates were 100% and 90.9% in the 3D and 2D groups, respectively, and only 25% in the 2D + 3D group. Furthermore, one patient underwent 2D brachytherapy, and the other one who received 3D brachytherapy developed distal metastasis. Of the six patients who received 2D + 3D brachytherapy, two (33.3%) developed distal metastasis, and one died, while two (33.3%) died of local recurrences.
Fig. 1
Kaplan-Meier curves showing the A) progression- free survival (PFS), B) overall survival (OS), and C) local control (LC) rates for 31 patients
There were no grade ≥ 3 acute or late gastrointestinal and urinary toxicities in the 31 patients. Toxicities of 2D and 3D brachytherapy are shown in Figure 2. A multivariate analysis was not conducted because the only variable that showed significance in the univariate analysis was the brachytherapy method for hematologic toxicity. In the univariate analysis, the brachytherapy technique and all other factors, including FIGO stage, chemotherapy, and external radiation therapy method, showed no significant difference in acute or late non-hematologic toxicity using chi-square analysis (Appendix Table 2); however, for late radiation toxicity, 3D brachytherapy had a lower prevalence of higher-grade toxicity, as presented in Figure 2.
Fig. 2
Comparison of acute and late toxicity between 3D-IGBT and 2D-BT. The outer circles represent 3D-IGBT, while the inner circles represent 2D-BT. Blue represents patients with no toxicity, green represents patients with grade 1 toxicity, orange represents patients with grade 2 toxicity, and red represents patients with grade 3 toxicity. A) Acute lower gastrointestinal toxicity, B) acute urinary toxicity, C) acute hematologic toxicity, D) late gastrointestinal toxicity, E) late urinary toxicity.
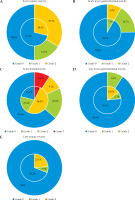
Table 2
D90% of clinical target volume (CTV) and D2cm3 of organs at risk (OARs) in patients who received 2D/3D brachytherapy
Dosimetry
Figure 3 shows a schematic diagram of dose distribution for the two patients who received 2D and 3D treatments. Among the 12 patients who received 3D-IGBT, the mean D90% value in 3D-IGBT was 76.5 Gy; four patients met the goal of D90% value to CTV ≥ 80 Gy, while all the patients met the OAR constraint. Of the 14 patients who received 2D brachytherapy, 12 with 17 fractions had CT images, which was helpful in reconstructing 3D plans to obtain their D90% values of CTV and D2cm3 values of OARs (Table 2). The mean CTV D90% value in 2D brachytherapy was 95.9 Gy, which was significantly higher than that in 3D brachytherapy (p = 0.005). Of the 12 patients who received 2D brachytherapy, seven met the goal of D90% value, seven exceeded the bladder constraint, four surpassed the rectal constraint, seven exceeded the sigmoid constraint, and six exceeded the small intestine limit. The average D2cm3 values for the bladder, rectum, sigmoid, and small intestine in 3D-IGBT were 77.81, 63.65, 66.54, and 64.27 Gy, respectively, while those in 2D brachytherapy were 140.00, 79.61, 79.11, and 108.08 Gy, respectively. Except for the rectum (p = 0.198), the D2cm3 values for the other OARs in 2D brachytherapy were significantly higher than those in 3D brachytherapy, showing p < 0.001 for the bladder, p = 0.030 for the sigmoid colon, and p = 0.017 for the small intestine.
Discussion
Although the effectiveness of 3D-IGBT in cervical cancer has been previously proven [12], the advantage of 3D brachytherapy over 2D brachytherapy for cervical stump cancer remains unclear. In 2021, Okada et al. reported a case of cervical stump cancer (T3bN1M0) successfully treated by combining external beam radiotherapy and CT-based IGBT [13]. Using tandem and ovoid applicators in brachytherapy, an EQD2 of 69.6 Gy was delivered to the patient for HR-CTV. The patient showed no evidence of recurrence or late adverse effects at 3 years and 8 months post-radiotherapy. However, this only case does not sufficiently prove that 3D-IGBT is a better choice to treat cervical stump cancer. In this retrospective study, we collected data from 31 patients over the past 10 years, and explored the efficacy and safety of 3D-IGBT in the treatment of cervical stump cancer.
In a study conducted between 1953 and 1977, 89 patients with cervical stump cancer were treated with EBRT and intra-cavitary brachytherapy. The 5-year PFS according to the stage of the patients with cervical stump carcinoma were 83.8% for stage I, 77.6% for stage II, 51.0% for stage III, and 37.1% for stage IV [14]. Another analysis in 1993 showed that PFSs for patients who received concurrent brachytherapy and external radiotherapy were 100%, 85%, 82%, 71%, 45%, 54%, and 30% in stages IA, IB, IIA, IIB, IIIA, IIIB, and IV, respectively [15]. In this study, for early-stage disease (FIGO stages IA, IB1, IB2, and IIA1), the 5-year PFS was 87.5%, and LC was 100%; for locally advanced cancer (FIGO stages IIB-IVB), the 5-year PFS was 63.8%, and LC was 70.5%. The LC was higher than that reported in a previous study on cervical stump cancer, partly due to the revolution in radiotherapy techniques in recent years. Compared with previous studies [16-21], the current study introduced intensity-modulated radiation therapy and concurrent chemotherapy to the patients, and demonstrated a significant survival benefit. IGBT has been reported to improve LC rates in cervical cancers [22, 23], which was also proven in the present study. Our results help to identify that technological revolution may also contribute to the clinical outcome of cervical stump cancer treatment.
According to the 2012 ABS guidelines for locally advanced cervical cancer, the recommended minimum dose of HR-CTV D90% for patients with an intact uterus is 80 Gy. However, there are no widely accepted criteria for patients with cervical stump cancers. Initially, we set a target dose of 80 Gy for the HR-CTV D90%; however, according to this standard, the actual dose was relatively low. Only four of the 12 patients who received 3D-IGBT and seven who received 2D-BT, met the initial goal. Our data analysis revealed that all patients who developed local recurrence received a target dose of < 75 Gy. If the goal was adjusted to 75 Gy, eight of the 12 patients who received 3D-IGBT and nine of those who underwent 2D-BT would meet the goal. Therefore, 75 Gy may be an appropriate dose criterion for the HR-CTV of cervical stump cancer, and could provide supporting evidence for further development of dose criteria for the treatment of cervical stump cancer.
One reason for obtaining a relatively low-dose in the CTV was the choice of reference points. Point A was defined as a point 2 cm above the vaginal fornix and 2 cm from the uterine axis according to the ICRU-38 report, which is widely accepted as the reference point to describe brachytherapy dose in patients with cervical cancer. However, when the original anatomical structure of cervical stump cancer is destroyed, point A may underestimate the dose of OARs. In this study, different reference points were applied to limit doses of OARs, which might lead to a relatively low-dose in CTV.
The current data show a higher exposure dose to OARs in 2D brachytherapy than in 3D-IGBT. This result is consistent with that of a previous study on cervical cancer with an intact uterine corpus [5]. The EMBRACE II study recommend a dose limit of 90 Gy for the bladder and 75 Gy for the rectum in patients with an intact uterus [10]. Concerning these criteria, of the patients who received 2D-BT, seven (58.3%) exceeded the bladder limit, and four (33.3%) exceeded the rectum limit, while all the patients who received 3D-IGBT met the constraint. In addition, the average doses to OARs in the 2D-BT group were significantly higher than those in the 3D-IGBT group. The above data show that 3D-IGBT might achieve better dose control in OARs than 2D-BT for cervical stump cancer. This effect further leads to a milder late toxicities of 3D-IGBT, with fewer grade ≥ 2 late gastrointestinal and urinary toxicities. Moreover, no grade ≥ 3 acute or late toxicity was found in the 2D-BT and 3D-IGBT groups, indicating good protection of OARs in our study. Unfortunately, chi-square test failed to demonstrate the significance of data, which may be attributed to the relatively small sample size. Nevertheless, the above evidence still suggests that 3D-IGBT, as opposed to 2D-BT, may be a better choice for the protection of OARs during radiotherapy for cervical stump cancer.
Tandem and ovoid or ring applicators are the most prominent applicators for cervical cancer. However, in clinical operations, due to changes in patient’s pelvic anatomy, it may be challenging to locate the cervix opening; therefore, the placement of an applicator could be problematic. For these patients, using cylinders may be a viable option; however, this may result in an insufficient apical dose [24]. Interstitial implantation is generally performed in patients with large tumors or in advanced stages. Previous studies have shown that combined intra-cavitary/interstitial brachytherapy can significantly improve LC in locally advanced cervical cancer [25], while using interstitial implantation for advanced stage cervical stump cancer requires additional data for analysis.
The main limitation of our study was that the long study period resulted in a wide variety of brachytherapy techniques, which hindered further analysis. In addition, this was a small sample retrospective study; the small sample size impacted the statistical analysis of some of the results, such as hematologic toxicity, reducing the reliability of the results. Moreover, not all fractions of 2D brachytherapy had reserved CT images, which may lead into a bias in the reconstruction results. However, our experience can guide the subsequent treatment of such patients. Further prospective, large-scale, multicenter clinical studies are required to validate the effectiveness of 3D-IGBT technique.
Conclusions
Compared with previous studies, we report a higher survival rate in patients with cervical stump cancer. Furthermore, 3D brachytherapy showed better outcomes in late toxicity and, for dosimetry, 3D brachytherapy delivered a lower dose to the bladder, rectum, sigmoid colon, and small intestine.