Introduction
The flow cytometry (FC) technique found its clinical application in the early 1980s. For almost four decades, the development and standardisation process of monoclonal antibody production, as well as the systematic progress in flow cytometer instrument design, including multiple excitation lasers and multiple parameters to be evaluated simultaneously, provided new clinical axnd diagnostic applications for this technique. The most popular application of FC is the diagnostics of haemato-oncological disorders, based on analysis of bone marrow (BM), peripheral blood (PB), or cerebrospinal fluid (CSF) samples. One of the most important advantages of FC is its speed in cell phenotype assessment, enabling determination of the cell lineage involved in malignant processes. Moreover, with the use of phenotypic markers specific for particular cell lineage, it enables distinction between reactive (normal) and malignant conditions, also during therapy monitoring [1].
Nowadays, trends in centralisation of diagnostics in highly specialised laboratories are observed in most countries. This is also true for FC-based diagnostics, and it is dictated on the one hand by the economic factors, and on the other hand it is a logical step to reduce site variability and to obtain a high level of efficiency, proficiency, reproducibility, and standardisation by batching the samples [2]. To maximise these factors in centralised networks, also when performing multicentre clinical studies, biological samples should be treated in the same way, which specifically restricts the time frame between material collection and the assay. It is widely known that prolonged storage of any biological specimen results in surface marker degradation, increase of cellular debris, loss of cell viability, increase in unspecific antibody binding, and cellular autofluorescence (Fig. 1) [3, 4]. The sample storage effect was investigated in several studies, but the conclusions were equivocal and clearly depended on material type, cell population of interest, and application [2, 5-13]. Also, different guideline manuscripts give ambiguous information [14-21]. Experimental data prove that the maximal storage time of unfixed PB or BM samples, ensuring optimal and accurate FC immunophenotyping at room temperature (RT), should be as short as 12 h or as long as 72 h depending on the investigated markers and applications [7, 14, 17, 22]. Refrigerating the samples at 4°C can further prolong the storage time and preserve the absolute counts of some cell types for four days and can provide acceptable results on expression levels (in median fluorescence intensity [MFI] units) of some antigens even after 7-10 days of storage [2]. Prolonged PB storage leads to selective loss of cell populations with shorter half-life, such as neutrophils and eosinophils, while lymphocytes and dendritic cells are the least affected [13]. In turn, according to the guidelines for CD34+ cell enumeration, the limit of 12 h at 4°C should not be exceeded [23]. Unfortunately, optimal time limit of sample storage and shipment might be difficult to reach in daily practice in larger countries or in developing countries with insufficient logistic infrastructure. Moreover, unintended shipping delays can occur, e.g. due to over-the-weekend transportation or around occasional holiday periods. On the other hand, a reliable cold-chain transport might not always be available, neither in local nor in international settings. Thus, to avoid the difficulties around sample storage and shipment that potentially compromise large scale multicentre studies, material stabilisation (fixation) should be taken into account as a universal solution. Alternatively, material deep-freezing (cryopreservation) might also be considered.
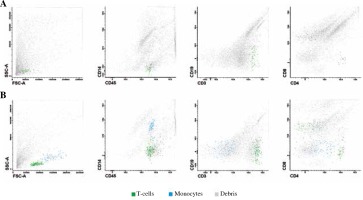
Fig. 1
Flow cytometric image of a native (unfixed) CSF sample (A) and TransFix®-preserved CSF sample (B). Both sample aliquots were acquired after 24-hour storage at 4°C. In the unfixed CSF sample significant loss of monocytes (blue) and lymphocytes (green) is visible accompanied by significant increase in cellular debris (grey) [own data]
The most widely used fixative agents are: paraformaldehyde (PFA), TransFix® (Cytomark, Buckingham, UK), and Cyto-Chex® (Streck Laboratories, La Vista, NE, United States). The two latter fixatives are available in liquid as well as in vacutainer forms, which allows direct blood sampling and cellular preservation [2, 4, 24]. The most abundant documentation is available for the usage of TransFix® that dates back to 1996, when it was used in the UK NEQAS external quality assessment schemes involving biological material dispatch. TransFix® was at that time reported to effectively preserve both the light scatter characteristics and antigenic profile of the samples [25, 26]. At that time, it also became clear that common accessibility of FC-based diagnostics (particularly in haemato-oncology) and establishment of national diagnostic networks require regular quality assessment (QA) programs, and TransFix® played a key role in the propagation of this important element of FC-based diagnostics. Besides successful usage within UK NEQAS programs, the application of TransFix® was assessed in many studies using different biological materials. In this review, we attempted to summarise the literature data on the influence of sample storage under different temperatures and times combined with different fixation conditions with the most popular fixative agents on the cell count and marker expression levels (in MFI units), which are the main determinants of the quality of samples dedicated for FC analyses.
Peripheral blood-based studies
Based on the findings of several extensive studies, it can be generally stated that the performance of the commonly used fixative agents greatly depends on the analysed marker and specific PB cell population expressing a given antigen [2, 5-7, 13, 27]. In the study of Ng et al., the effectiveness of TransFix®, Cyto-Chex®, and PFA (in two concentrations of 1% and 4%) in flow cytometric PB analyses was assessed [2]. The results showed that the preservation of cell surface marker expression (given in MFI units) was strongly marker-dependent (Table 1). Overall, TransFix® turned out to be the best fixative reagent for surface markers, being superior to Cyto-Chex® and PFA in the preservation of MFI of such markers as CD11b, CD19, CD4, CD45, CD66, and CD86. Conversely, Cyto-Chex® was better than TransFix® and PFA in preserving the MFI of such antigens as BDCA2 (CD303), CD25, and CD3. In turn, three markers: BDCA3 (CD141), CD123, and CD80 were equally well preserved by all assessed fixatives. Notably, none of the PFA solutions was superior in preserving the MFI of any specific population or marker other than Cyto-Chex® and TransFix®, with exception of the intracellular marker FOXP3 (Table 1). Of note, the MFI of CD8, BDCA1 (CD1c), CD11c, CD20, CD49d, and NKP46 with addition of either of the studied fixatives was not found to be significantly better preserved as compared to unfixed samples [2].
Table 1
Summary of storage/shipment conditions required for acceptable preservation of marker expression and cell count assessed in peripheral blood
| Characteristic | TransFix® | Cyto-Chex® | Paraformaldehyde (PFA) |
|---|---|---|---|
| Optimally preserved marker expression (MFI) | CD11b, CD19, CD4, CD45, CD66, CD86: 10 days at RT [2] | BDCA2 (CD303), CD25, CD3: 10 days at RT [2];CD11b on granulocytes: 4 days storage at RT [31] or 7 days storage at 4°C [30] | Intracellular FOXP3: 10 days at 4°C [2] |
| BDCA3 (CD141), CD123, CD80: for 10 days at RT for Transfix® and Cyto-Chex® and at 4°C for PFA [2] | |||
| CD127: 10 days at RT for Cyto-Chex® and at 4°C for PFA [2] | |||
| CD45RA, CD56: 10 days at RT [2] | No data | ||
| At RT: CD19: 4 days; CD45: 42 h At 4°C: CD3, IgM: 72 h; CD19: 24 h; CD45, IgD: 42 h [13] | At RT: CD45: 24h; CD45RA: 72 h [13] | No data | |
| Optimally preserved cell count | BDCA1+ mDC, BDCA3+ mDC, pDC, CD8+ T cells: 10 days at RT [2] | CD16+ monocytes, total T cells, NK cells, granulocytes, BDCA3+ mDC, pDC: 10 days at RT [2] | No data |
| Total T-cells (CD3+, CD4+, CD8+), NK cells (CD16+/CD56+), B cells (CD19+): 15 days [4] | |||
| Allowable transport/storage time | 10-14 days at 4°C or 5-10 days at 25-37°C [27] | 2 days: preservation of > 90% granulocytes count or 7 days: preservation of > 45% granulocyte count [31] | No data |
| Up to 10 days at 23-31°C [2] | |||
As concerns absolute cell counts, the majority of the evaluated cell populations was best preserved in Cyto-Chex®-fixed PB samples. The preservation obtained with TransFix® was better only for BDCA1+ (CD1c+) and BDCA3+ (CD141+) myeloid dendritic cells (mDC), plasmacytoid DC (pDC), and CD8+ T-cell subpopulation. For none of the evaluated cell populations PFA solutions performed better than Cyto-Chex®, TransFix®, and unfixed samples [2] (Table 1).
The same authors compared also the impact of PB sample transportation on cell count and marker expression level with the addition of TransFix® and Cyto-Chex® vs. unfixed samples, all shipped at an ambient temperature of 23-31°C. The results showed that the shipment of PB samples (total time lapse of 10 days) fixed with either of the fixative reagents did not have a major impact on cell count as compared to corresponding fixed, unshipped samples. It was also shown that the shipment of PB samples fixed with either fixative significantly outperformed shipment of unfixed samples in cell count preservation. When it comes to the assessment of marker expression stability, samples that were shipped after TransFix® fixation exhibited reduced CD4 expression on T cells and CD14 on monocytes, as compared to the freshly assayed samples. Shipment of samples without prior fixation had a detrimental effect on identification of specific cell populations, accompanied by slightly decreased expression of the majority of the assessed markers, with the exception of BDCA1 (CD1c), BDCA3 (CD141), CD8, and CD80. However, marker expression was best preserved in the absence of both shipment and fixation [2].
The three aforementioned fixative agents were also evaluated in an extensive study by Diks et al., based on long-term analysis of the expression of cell surface antigens composing the EuroFlow-designed tube for primary immunodeficiencies detection (PIDOT) [13]. PB samples fixed with TransFix® (stored at RT or 4°C) and Cyto-Chex® (stored at RT) exhibited an initial decrease in MFI (of 10-30% as compared to fresh PB samples) of approximately half of the 11 evaluated antigens in each condition (CD4, CD8, CD16+CD56, CD27, TCRᵞᵟ in samples fixed with Cyto-Chex® at RT; CD8, CD16+CD56, CD27, TCRᵞᵟ, CD45RA in samples fixed with TransFix® at RT or 4°C). In all cases CD16+CD56 on NK cells and CD27 on T-cells were the most affected (> 30% decrease in MFI as compared to fresh PB samples). Prolonging the storage time to 30 h or more (up to 14 days for Cyto-Chex® at RT and TransFix® at 4°C and up to four days for TransFix® at RT) resulted in a significant decrease of MFI of all examined markers in Cyto-Chex® tubes and most markers in TransFix®-treated samples. The most stable expression was observed for CD45 in samples fixed with Cyto-Chex® (24 h) and with TransFix® at RT or 4°C (42 h; Table 1). The authors also evaluated the relative distribution of main leukocyte subsets, concluding that both Cyto-Chex® and TransFix® caused gradual loss of granulocytes and overrepresentation of lymphocytes and monocytes after 24-hour storage at RT [13], which is concordant with observations of other authors [5] and reproduces the trend observed in unfixed PB samples stored at 4°C [13]. The authors also demonstrated that the addition of PFA to the PB previously fixed with FACS Lysing Solution used for erythrocyte lysis did not have any additional beneficial effect on the stability of the assessed cell surface markers of PIDOT tubes [13].
Another study by Canonico et al. [27] extended the range of time and temperature of sample storage. Both these factors, together with cell density, influenced the degree of morphological and phenotypic changes in TransFix®-preserved PB specimens transported for prolonged periods. It turned out that TransFix® allows transportation of samples for a period of 10-14 days if maintained at 4°C and for 5-10 days if maintained in suboptimal conditions, comprising both increased temperature (between 25°C and 37°C) and high cell density. The results of this study show that the quality of lymphocyte staining for CD3 and CD4 in fixed samples seems not to be affected even after 15-day storage at 37°C; however, lower temperatures (between 4°C and 25°C) are preferred because higher temperatures tend to increase the autofluorescence of different cells, which was also confirmed by Harrison et al. [4]. In contrast, monocytes exhibited a rapidly decreasing MFI of CD4 staining over time, which confirms greater stability of lymphocytes in TransFix®-preserved samples [5, 27]. Importantly, it was also demonstrated that TransFix® can prevent apoptosis and its addition stabilises cell ultrastructure suppressing the degenerative cellular processes in leukocytes, both necrotic and apoptotic. To prove this, assays such as TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labelling), FC DNA content evaluation, and DNA gel electrophoresis were performed to reveal the absence of apoptosis-related oligonucleosomic strand breaks in TransFix®-preserved samples [27]. This finding might be of potential use for studying proapoptotic capabilities of certain drugs or other substances in samples subjected to prior fixation.
The study involving leukocyte staining by Canonico et al. proved that the use of TransFix® stabilises PB leukocytes for at least 10 days and preserves their light scatter characteristics, immunophenotype, and absolute counts [5]. Only at the end of the investigated TransFix®-preserved PB sample storage period (10 days) was cell morphology slightly altered, but with no influence on absolute cell counts and expression levels of CD45, CD3, and CD38 antigens, as compared to fresh, untreated specimens. It was again shown that of all leukocyte subpopulations, lymphocytes were better preserved by TransFix® addition than were monocytes and granulocytes. This was demonstrated in four ways: (1) monocytes and granulocytes showed more significant fluorescence drop of the studied antigens between day 0 and day 10 (17% and 30% decrease in MFI, respectively); (2) monocytes and granulocytes generated slightly lower forward (FSC) and side scatter (SSC) that was visible 1-2 days after fixation for granulocytes and after 4 days for monocytes, but without generation of separate populations of dead cells of significantly reduced FSC, as visible in non-fixed samples; (3) granulocytes and monocytes exhibited a tendency towards propidium iodide uptake greater than lymphocytes and increasing with time, as an effect of possible TransFix®-induced alterations in membrane permeability, supported also by; (4) ultrastructural morphology studies with transmission electron microscopy [5]. A higher vulnerability of granulocytes in the samples with addition of TransFix® was also confirmed in another study by Canonico et al., in which the authors also showed that TransFix®-treated whole blood can be used for delayed CD4+ T cell enumeration [27]. The effect of TransFix®-induced cell membrane permeabilisation, which was detrimental to intracellular marker identification was also proven by increased intracellular propidium iodide uptake reported by Canonico et al. [5]. This was also demonstrated by Ng et al., who illustrated this effect by permeabilisation assay with 7AAD staining [2].
The ability of TransFix® and Cyto-Chex® to preserve the counts of lymphocyte subpopulations as determined with BD Multitest IMK kit (BD, San Jose, CA), containing monoclonal antibodies against CD19, CD45, CD3, CD4, CD8, and CD16+CD56 was assessed by Harrison et al. [4]. The results showed that PB samples collected with dedicated TransFix®- and Cyto-Chex®-containing blood collection devices after a 15-day storage period provided highly comparable results of enumeration of CD4+ T-cells in HIV-infected patients to those obtained from freshly collected PB. The same was also true for the remaining lymphocyte subpopulations assessed in healthy donors [4]. The expression of lymphocyte surface antigens was preserved over 15 days of storage; however, the MFI of all of the assessed antigens was significantly lower as compared to a fresh reference sample. In direct comparison, TransFix® turned out to perform better than Cyto-Chex® in marker MFI preservation [4], which is in line with the findings of Ng et al. cited above [2].
TransFix® was also successfully used for delayed CD34+ stem cell enumeration within UK NEQAS programs [23, 28]. Similarly, CD34+ endothelial progenitor cells and angiogenic T-cells assisting in endothelial repair processes were shown to be reliably enumerated in TransFix®-preserved whole blood stored at RT for a period of up to seven days [6, 29].
Davis et al. performed a study evaluating the stability of the percentage of lymphocyte subpopulations in Cyto-Chex®-fixed PB samples vs. non-fixed, all stored at RT for seven and two days, respectively [24]. It was demonstrated that percentages of lymphocytes expressing CD3, CD4, CD8, CD3+CD45RA, CD3+CD45RO, CD19, and CD16+CD56, as well as chemokine receptors CCR5 and CXCR3, can be accurately determined up to seven days after blood collection in Cyto-Chex®-fixed samples. However, the percentage of T cells expressing the CLA antigen in fixed samples was about 50% lower than that observed in freshly collected blood, but this decreased expression was stable until the end of the seven-day evaluation period. The stability of activation markers CD25 and HLA-DR on T cells did not appear to improve in fixed blood, and their expression was found to decrease strongly after four days [24].
In yet another study by Elghetany et al., the impact of Cyto-Chex®-fixation on PB granulocyte viability and specific markers expression was assessed. After two-day storage 90% of the initial granulocytes were viable, whereas seven-day storage resulted in 45% recovery of the initial granulocyte number. Moreover, the MFI of CD11b remained stable throughout the observation period, while the MFI of CD16, CD18, and CD44 was slightly decreased [7]. Similar observations on the applicability of the Cyto-Chex® for delayed granulocyte studies with successful CD11b assessment were also performed by other authors [30-32].
Based on the above summarised examples, the selection of a particular fixative agent depends on the aim and design of the study, which determines what is more important to preserve: cell count or marker expression stability. It should be also optimised to the cell type(s) and the specific surface or intracellular antigens that are to be assessed. It is also important to note that any biological activity studies require viable cells, so fixatives that potentially prevent apoptosis, like TransFix®, should be preferred [27]. Some technical aspects might also be important when choosing the proper fixative. For example, PFA causes also fixation of red blood cells, which precludes their lysis after leukocyte staining. Therefore, it is recommended that samples dedicated for transportation should first be stained and fixed with PFA only afterwards. However, this is not the case for TransFix® and Cyto-Chex®, which can be safely added before transportation and staining with no influence on subsequent ability to lyse red blood cells [2] (Table 1). It was also shown that PFA is not suitable for granulocyte-based studies because it (1) prevents their responsiveness to chemotactic/activating agents such as N-formylmethionyl-leucyl-phenylalanine (fMLP) and (2) damages epitopes of some granulocytic surface antigens, such as CD13, CD32, and CD62L [7]. However, delayed studies involving granulocytes, as well as remaining leukocyte subsets, can be significantly improved by cryopreservation. It was elegantly shown by de Ruiter et al. that fixation of leukocytes and lysis of erythrocytes in PB samples (with the use of FACS Lysing Solution, BD) followed by deep-freezing at –80°C in RPMI medium supplemented with dimethyl sulfoxide (DMSO) and foetal calf serum yields highly comparable results concerning the expression of CD11b, CD66b, CD35, and CD62L on neutrophils, CD193, CD66b, CD35, CD11b, CD62L, and CD69 on eosinophils, and CD11b, CD35, and CD203c on basophils, to both fresh and fixed cells that were not subjected to cryopreservation. It was also proven that the differences in expression levels, as well as in light-scatter characteristics, were mainly caused by fixation alone and not the freeze-thaw process. Furthermore, neither fixation alone nor fixation and cryopreservation impeded the determination of in vivo activated neutrophils, which also retained their responsiveness to in vitro activation by fMLP, as compared to fresh cells, even after freezing at –80°C for as long as two years [33]. Nemes et al. described several functional assays employing fixed and cryopreserved cells, such as cytokine production profile, cytotoxic potential, or determination of proliferation of T-cells [34]. The same investigator also showed that fixation and cryopreservation of PB samples enabled accurate FC assessment of percentages of different leukocyte subsets: granulocytes, monocytes, T-cells, B-cells, and NK-cells. The selected lineage markers used for the definition of these subsets exhibited similar (CD19) or higher (CD3, CD14, CD45) expression levels in cryopreserved as compared to fresh samples. Contrastingly, absolute cell counts were slightly underestimated in cryopreserved vs. fresh samples, which was dependent on cell type (the greatest difference observed for granulocytes) but independent on time of cryopreservation (up to 1 year) [34]. Based also on the findings of other authors, the greatest differences between cells enumerated in fresh and cryopreserved PB samples were observed for the relatively rare cell subpopulations, such as CD56-bright NK-cells, TLR4+ monocytes, terminally differentiated CD4+ T-cells, or Ki-67+ T-cells [35].
Cerebrospinal fluid-based studies
From the available literature data, for CSF-based studies requiring sample stabilisation, only TransFix® and serum-containing media were used. FC is a frequently used method for the identification of leptomeningeal localisations of haematological malignancies. For that purpose, CSF is subjected to analysis in all patients suspected of such medical conditions as primary central nervous system lymphomas (PCNSL), patients with other haematological malignancies complicated by neurological signs and symptoms suggestive of meningeal involvement, patients with aggressive non-Hodgkin lymphomas (NHL), and in cases of leptomeningeal acute leukaemia relapses [8, 10, 36-38]. Since the FC was incorporated as a supplementary technique to cytomorphological CSF assessment, the detection rate of CSF involvement has risen to 86%, higher than cytomorphology alone [10]. For example, cytomorphological CSF involvement in acute leukaemias is usually detected in approximately 10% of patients, while FC suggests CSF leukaemia in more than 20% of patients [39]. CSF is a very perishable material, and the primarily low cell number in CSF (usually < 5 cells/ml) rapidly decreases ex vivo (Fig. 1) [9, 40]. This phenomenon may lead to a false diagnosis of patients primarily presenting with normal cellularity or mild pleocytosis in which the time elapsed between lumbar puncture and analysis might have influenced CSF sample cellularity [9]. Most FC assays involving CSF require cell concentration by proper centrifugation, which also contributes to unwanted cell loss and impaired cell recovery [10, 40]. Optimal conditions for CSF handling prior to FC analyses were described elsewhere [10, 40]. Nevertheless, CSF samples intended for FC analyses should be processed immediately after withdrawal, to minimise cell loss and sensitivity reduction [10, 11]. To prolong cell viability in CSF samples, serum-containing media can be used, which was proven to prevent cellular loss during up to at least 5 h of storage [9, 10]. On the other hand, according to guidelines by Johansson et al., storage of CSF at 4°C in fixative reagent or culture media is possible for a maximum of 24-48 h [14]. However, tubes with serum-containing medium are not commercially available and have a limited shelf life of around three months. Therefore, an attractive alternative is TransFix® CSF storage tubes because of their commercial availability and longer, one-year shelf life [8]. TransFix® in combination with ethylenediaminetetraacetic acid (EDTA) is widely used to stabilise CSF samples to enable overnight shipping to a central flow cytometry facility [36].
In a study performed by de Jongste et al., the effect of TransFix® on the detection of haematological malignancies in CSF and CSF cell numbers by FC was investigated [8]. CSF samples were either processed immediately (up to 30 minutes after withdrawal) or after overnight, 18-hour storage in TransFix®. For comparison, simultaneously collected CSF samples stabilised with serum-containing medium and native CSF containing no cell-stabilising agents were used. Assessment of sample quality 30 minutes after withdrawal did not reveal significant differences between native CSF and TransFix®- or serum-containing media CSF samples. Conversely, after 18-hour storage, the use of TransFix® significantly enhanced malignancy detection as compared to CSF with serum-containing medium and native CSF samples. As for quantitative assessment, 30 minutes after CSF withdrawal, the median absolute number of all leukocytes in TransFix®-preserved samples was similar to those preserved with serum-containing medium but 1.4 times higher than in native CSF. In contrast, after 18-hour storage with the addition of TransFix®, the median leukocyte number in CSF samples was 1.8 times higher than in CSF with serum-containing medium and 2.3 times higher than in native CSF. Higher absolute leukocyte counts in TransFix®-preserved CSF samples resulted from higher lymphocyte counts, suggesting that lymphocyte numbers in the CSF of patients decrease at higher rate as compared to monocytes and granulocytes [8]. This finding was, however, contradictory to the phenomenon reported earlier by Dux et al., that lymphocytes in CSF were the most resistant cell population to storage time [40]. Based on the results of the study of de Jongste et al., fixation of CSF samples in TransFix® causes a slight but significant decrease in MFI of four out of the five assessed B-cell markers (sIgκ, CD19, CD20, CD45) as compared to CSF with serum-containing media and/or native CSF, even after 30-minute sample storage. Only sIgM staining was not sensitive to the addition of any of the fixatives. As concerns the light scatter characteristics, it was demonstrated that the diffuse large B-cell lymphoma cells had reduced forward and side scatter properties in CSF samples with TransFix®, as compared to specimens with serum-containing medium and native CSF samples [8].
The results of another study show that the addition of serum can significantly improve the CSF sample quality and prolong its usefulness for the analysis [9]. The counts of different leukocyte types were compared between native CSF and CSF with serum-containing medium at different time points ranging from 30 min to 5 h. It turned out that at all time points, the counts of all types of leukocytes in native CSF samples were significantly lower than in CSF with serum-containing medium, except for the granulocyte count at 30 minutes, which remained stable [9]. The most vulnerable cell type were monocytes, which exhibited the highest degree of reduction 1 h after sampling, followed by lymphocytes and granulocytes. At later time points further cell count reduction was observed, which was the most pronounced for monocytes, followed by lymphocytes. In contrast, there were no significant differences between granulocyte counts at 1 and 5 h, which is at least partly contradictory to the observations of other studies [8, 40].
The influence of the addition of serum-containing medium was also studied by Greig et al. [12]. They found that the use of a native, non-stabilised CSF for flow cytometric studies yielded around 30% of cases not having an adequate number of viable cells with the remaining 70% of cases had either insufficient quantity or specimen viability below analytical limits for study. However, when the CSF was stabilised with serum-containing medium, more than 90% of samples yielded adequate numbers of viable cells that were suitable for flow cytometric analysis [12].
In conclusion, the fixation of CSF either with TransFix® or with serum-containing medium prevents cellular loss and enhances FC-based detection of leptomeningeal localisations of haematological malignancies, even after 18-hour sample storage. The use of TransFix® may also facilitate FC analysis of CSF, especially when the samples are collected outside office hours or require shipment to an external (central) laboratory with FC facilities. Additionally, TransFix® may cut costs via the introduction of batch-processing of CSF samples instead of immediate processing of single samples [8]. Finally, the use of fixative agents brings the advantage of gaining time, which is more important than slight fixative-dependent reduction of MFI of some markers or decreased light scatter characteristics, neither of which cause CSF sample disqualification for FC assay.



