Purpose
Eyelid tumors originate from the skin and sebaceous glands, and account for approximately 3% of all head and neck tumors, with 5-10% of cutaneous malignancies. The common histologies in eyelid tumors are basal cell carcinoma (BCC) from the skin accounting for 75% of cases, while the other 25% comprise of squamous and adenocarcinoma or sebaceous cell carcinoma from the meibomian glands [1]. They are usually high-grade tumors, necessitating combined modality treatment. Surgical excision with adequate negative margins is the most important prognostic factor for favorable outcomes. In patients with R0 resection, adjuvant radiotherapy of 60 Gy in 2 Gy equivalent dose (EQD2) may be indicated for high-grade tumors with close or inadequate margins, lymphovascular space invasion, perineural invasion, and positive lymph nodes [2, 3]. Positive surgical margins include residual microscopic (R1) or macroscopic (R2) disease, in which re-excision is not feasible. Such cases necessitate post-operative salvage radiotherapy to a dose of 66 Gy EQD2 [3]. Radiation delivery using external beam radiotherapy (EBRT) to the eyelid is limited by the presence of critical structures, such as the orbit, lens, retina, and optic nerve. Therefore, the ideal technique for delivering post-operative adjuvant radiotherapy for eyelid tumors is interstitial brachytherapy (ISBT).
Brachytherapy, also known as interventional radiotherapy, is the most conformal technique of radiation delivery, strategically avoiding unnecessary exposure of surrounding critical structures. However, its use is limited in eyelid tumors due to the steep learning curve.
The current paper intended to describe our procedure of ISBT application to the eyelid in a stepwise fashion, performed in four patients with eyelid tumors, with their short-term clinical outcomes reported.
Case description
Four patients with sebaceous carcinoma of the upper eyelid were referred for adjuvant radiotherapy post-wide local excision. Three female and one male patients had a localized disease, with no nodal metastasis, while two out of the four patients had positive surgical margins. A common problem encountered among all four patients was a delay in diagnosis, as the lesions were wrongly identified as a chalazion or hordeolum. The average duration from symptoms to diagnosis was six to eight months. As a result of low index of suspicion for malignancy, they were surgically excised without pre-operative imaging. Patients were referred for adjuvant managaement after obtaining histopathological evidence of malignancy. This posed challenges with respect to time to initiate adjuvant radiotherapy as well as deciding upon implant volume. The implant volume was planned based on the extent of tumor described by the patient and the surgeon. The first pre-requisite to perform ISBT of the eyelid is to understand the anatomy of the upper and lower eyelids.
Structure and function[4]
The skin of the eyelid is about 1 mm thick with no subcutaneous fat, and has the thinnest skin on the body. Orbital septum separate anterior structures of the eyelid from intra-orbital contents. Tarsal plates, the main structure of the eyelid, contain dense connective tissue, meibomian glands, and follicles of eyelashes along with Zeiss and Moll glands that secrete lipid or sweat. The tarsal plate of the upper eyelid is 10-12 mm vertically, while the tarsal plate of the lower eyelid has a central length of 4 mm vertically. The skin of the eyelid is loosely attached above the tarsal plates, thus allowing a potential space beneath. There are approximately 30 meibomian glands in the upper eyelid and 20 meibomian glands in the lower eyelid. Moreover, there are approximately 100 eyelashes in the upper lid in 2 to 3 rows, and 50 eyelashes in the lower eyelid. The conjunctiva lines the inner surface of the eyelids, and extends in continuity over the anterior surface of the globe up to the cornea. The eyelids play a protective and lubricative role over the ocular surface, by ensuring adequate tear film distribution across the eye as well as cosmesis.
Tumors may arise from any of these above-mentioned components of the eyelid. Its thin skin with relatively little subcutaneous connective tissue allows rapid local invasion of tumors. Therefore, post-operative recurrent lesions can be very invasive, suggesting a lower threshold of adjuvant radiotherapy in cases with close margins post-surgical excision.
Procedure of brachytherapy catheter placement
Patients were admitted one day prior to the procedure, after obtaining pre-anesthesia clearance. The procedure was performed under a short general anesthesia. The skin over the upper and lower eyelids was painted with povidone iodine solution. Single-plane catheter placement is usually sufficient to cover eyelid tumors, owing to a sub-centimetric thickness of the tissue. Entry and exit points were drawn based on the size and extent of the tumor, providing a margin of 0.5-1 cm. A hollow ISBT needle with a stylet was inserted into subcutaneous plane of the eyelid, taking utmost care not to penetrate through the thickness of the eyelid, or injuring the eyeball (Figure 1A). In order to avoid traction-induced trauma and excess edema of the eyelid, it was essential to provide counter pressure over the eyelid with a gauze while withdrawing the catheters after removal of the needles. Care was taken not to insert the needle in the tarsal margin or within 2 mm from the tarsal margin to avoid its contractures.
Fig. 1
A) Needle placement for eyelid brachytherapy. B) Replacement of needle with a nylon catheter. C) Insertion of multiple catheters to cover the tumor bed with planned margins. The catheters were pulled gently such that the buttons would abut the skin at the time of obtaining the planning CT scan
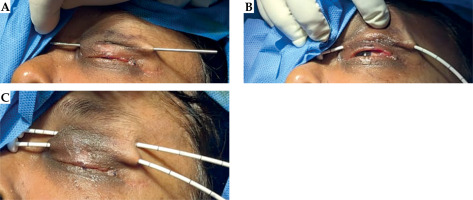
To ensure optimal placement, the most inferior needle was inserted first, followed by the next cranial needle, with suitable gap between the two (2-3 needles would generally be sufficient to cover the tumor bed adequately). The needles were then replaced with 6 Fr flexible catheters by threading the catheters in the hollow needles and withdrawing the needles, thus leaving the catheter in situ (Figure 1B, C). Although catheters thinner than 6 Fr may be less invasive, they were not available at the time of procedure. The catheters were fixed with soft buttons and positioned at the end of the surgery to allow a 5 mm gap from the skin for post-procedural edema. Then, exiting ends of the catheters were gently coiled and secured by wrapping them with a gauze pad, and attaching it to the side of the head with a tape. A short course of intravenous dexamethasone 4 mg was administered twice a day for 1-2 days, under the cover of proton pump inhibitors, for eyelid edema to resolve completely before CT simulation.
Acquisition of CT scan planning
Planning CT scan was performed on day 2-3, after post-procedural edema subsided. Prior to the scan, the catheters were adjusted such that the buttons gently abutted the skin, thus fixing the treatment length. Patients were scanned with a 16-slice Philips wide bore helical CT scanner, using the following imaging protocol: 40 mA and 120 kVp settings, vertex to mentum, with axial slices of 1 mm thickness obtained without contrast.
Brachytherapy planning
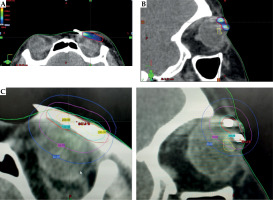
Planning was performed with BrachyVision planning system (version 13.7, Varian Medical System, Palo Alto, California, USA). A plan was generated using equal dwell times followed by manual optimization, normalized to 90% isodose line. 200% isodoses were strictly limited to the catheters. 3.5 Gy in 12-14 fractions were planned to deliver an adjuvant EQD2 dose of 60 Gy for patients with R0 resection and 66 Gy for R1 resection (Figure 2A, B).
Treatment delivery
The catheters were numbered on the first day to avoid errors during connection to HDR unit. Two fractions were delivered daily, with a minimum of 6 hours interval. In order to appropriately shield the 0.4 MeV average energy photons from iridium-192 (192Ir) source, a lead shield with wax coating of 2-3 mm was prepared to protect the cornea, conjunctiva, and lens (Figure 3). The eye was first anaesthetized with 0.4% paracaine eye drops, and the lead shield was placed over the cornea and conjunctiva under the eyelid in anesthetized eye.
Dosimetry
In vivo dosimetry was performed using metal-oxide-semiconductor field-effect transistor (MOSFET; Best Medical, Canada).
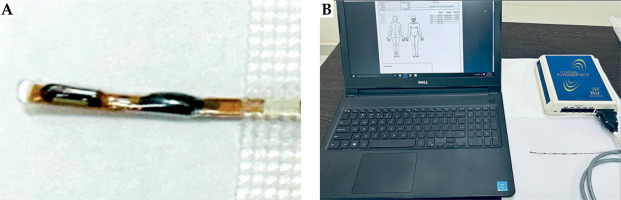
The mobile MOSFET is an electronic device that measures the radiation dose. It features a silicon chip measuring 1 mm × 1 mm, with an active area of 0.2 mm × 0.2 mm placed beneath a black epoxy bulb, located at the end of a flexible cable (Figure 4A). The change in the voltage caused by irradiation is proportional to the absorbed radiation. The wireless transceiver acts as a channel between the reader module and remote verification software (Figure 4B). TPS uses dose calculations based on 1 mm CT slice thickness, whereas MOSFET, which is thinner than 1 mm, allows a measurement of point dose. Thus, the TPS measures the average dose, while the MOSFET measures the maximum dose received by the ocular surface beneath the shield.
Fig. 4
A) Mobile metal-oxide-semiconductor field-effect transistor (MOSFET). B) The dose recording and verification system
The reader module was adjusted to standard bias setting with sensitivity of 1 mV/cGy. Prior to its use, the MOSFET was calibrated, for which, dosimeters were exposed to low doses (range, 0.5-5.0 cGy) with 0.5 cGy increments, using 192Ir source. Each measurement was taken three times, and the average was adopted in the study. A calibration curve was created by plotting the MOSFET response (mV) versus the dose (cGy).
Recording was performed at the first, third, and fifth fractions. The mobile MOSFET was gently placed on the anaesthetized conjunctiva, upon which, the shield was placed such that the MOSFET was sandwiched between the corneal shield and the ocular surface. To ensure reproducibility of measurement, a small mark was made on the MOSFET cable and placed in such a way that the mark was at the lateral canthus of the eye, each time of the measurement.
Catheter removal
Under aseptic precautions, the buttons were gently loosened at the open end. The fixed end was cut, and all the catheters were removed in a single sweeping motion, with counter pressure applied on the eye simultaneously. A gentle compression dressing was provided after the removal. Patients were discharged after one day of observation post-removal of the catheters. The total duration of hospital stay was limited to approximately 15 days.
Results
Three out of four patients were females. The median age was 54 years (IQR, 42.5-63.5 years), and the mean CTV volume was 2.1 ±1.2 cc. The minimum dose received by 90% of CTV (D90) was 3.37 ±0.07 Gy per fraction. The target V100% was 86.27 ±2.22%, V150% was 30.50 ±5.2%, and V200% was 11.33 ±5.8%. The lens Dmax (TPS) was 1.28 ±0.58 Gy per fraction. The mean dose recorded by the MOSFET was 0.69 ±0.10 Gy per fraction. No acute toxicities were observed. Grade 1 skin reaction of the Radiation Therapy and Oncology Group (RTOG) grading was observed in the form of hyperpigmentation in all four patients. None of the patients experienced conjunctival erythema or corneal edema. At a median follow-up of one year, no local recurrences were observed. Late reactions were mild hyperpigmentation seen in all four patients.
Discussion
Eyelid tumors are often managed by surgical excision, such as Moh’s micrographic surgery or wide local excision. Adjuvant radiotherapy is indicated in tumors with high-risk of local recurrence, such as high-grade histology, and close or positive surgical margins [2]. Tumor control and functional and cosmetic outcomes are the main goals of treatment in eyelid tumors, best achieved by delivering adjuvant radiation using brachytherapy. External beam radiotherapy (EBRT) can lead to several acute adverse effects with progressively increasing doses. At 30 Gy, acute effects may occur, including erythema of the eyelid, conjunctival congestion, epiphora, and loss of eyelashes at 40 Gy. This may further progress into late effects, such as ectropion, entropion (at doses above 50 Gy), corneal ulceration (above 60 Gy), cataract (at doses as low as 10 Gy), and retinal and optic nerve tolerance (at 45 Gy and 54 Gy, respectively). Frequently, these high doses are a problem during delivery of radiation using EBRT and brachytherapy alone.
Interventional radiotherapy may be delivered as interstitial brachytherapy or surface mould brachytherapy. It enables the delivery of a dose equivalent to 60 Gy without any of the above-mentioned adverse effects. Evidence reveals lesser usage of HDR brachytherapy in small groups of patients (range, 8-20) [5-9], whereas greater experience is shown using low-dose-rate (LDR) brachytherapy [10]. However, technical details of an ISBT procedure have been occasionally elaborated. The current report describes the procedure of ISBT used for eyelid tumors.
In India, Laskar et al. [6] reported their experience of ISBT in eyelid tumors in 8 cases, with excellent cosmetic and functional outcomes. In all patients, grade 1 toxicity by RTOG scoring was observed in the form of faint erythema and mild epiphora. The authors proposed the utilization of an indigenously designed toxicity scale, known as cosmesis after interstitial brachytherapy (CAIB) scale. It consisted of late effects, including depigmentation of the skin, eyelid dysfunction, such as ectropion or entropion, dry eye, keratitis, cataract, and glaucoma. Two of the 8 patients were reported to experience late toxicity in the form of persistent pigmentary changes and mild ectropion.
We had similar results in terms of local control and toxicities, with 4/4 grade 1 toxicity and no grade 3/4 toxicities in any patient. In addition, we used corneal shield and in vivo dosimetry with the MOSFET to document doses to the cornea and underlying structures. The corneal shield, when applied beneath the eyelid, reduced the mean dose to the underlying cornea to 0.69 ±0.10 Gy/fraction. Hence, even though the TPS demonstrated the mean lens Dmax of 1.28 ±0.58 Gy/fraction, the dose received by the lens under the shield was lesser than 0.69 ±0.10 Gy/fraction, allowing a reduction of dose by 46%.
Vavassori et al. [9] treated 10 eyelid malignancy patients using high-dose-rate brachytherapy (HDR-BT) with a customized mould applicator. Their patients demonstrated excellent local control and cosmetic outcomes at 4 years, with no epiphora or visual impairment.
The beauty of brachytherapy is best defined by a rapid dose fall-off according to the inverse square law. Cisek et al. [5] used brachytherapy in 28 patients as the only treatment modality. They treated their patients as per 2 irradiation schedules: 49 Gy/14 fractions at 3.5 Gy twice a day (9 patients), and 45 Gy in 5 Gy fractions twice a day for 5 days (19 patients). The lens, retina, cornea, and optic nerve received 18.2, 12.6, 48.6, and 10.8 Gy, respectively, in the first schedule, and 17.1, 11.7, 44.1, and 9.0 Gy, respectively, in the second schedule. These reports, however, have not described the utilization of a corneal shield. Mareco et al. [7] used lead shields to protect underlying structures in their 17 patients treated with HDR-ISBT. However, they reported 8 of the 17 patients (46%) developing keratoconjunctivitis sicca while receiving ≥ 4.5 Gy per fraction. Moreover, 7 of the 17 patients (42%) developed chronic conjunctivitis, which were more common in those treated for recurrent lesions. Madarosis was a common late adverse effect observed in 11 of the 17 patients (65%), due to the higher V100% sought in their study.
Dose estimation with the use of corneal lead shield may not be feasible with a planning system due to heavy artifactual effect in planning CT scan. Therefore, we used the MOSFET to measure the dose to the cornea. This ensures that doses delivered to structures beneath the cornea are definitely less than that measured at the level of the cornea.
A close proximity of applicators may inadvertently contribute to a high corneal dose. Studies showed the risk of corneal damage occurring beyond a conventionally fractionated external beam radiotherapy physical dose of 50 Gy as well as the risk of corneal ulceration after exceeding 60 Gy [11].
The severity and timing of cataracts are dose- and time-dependent. Research demonstrated a 33% cataract risk within 8 years when the lens receives a dose in the range of 2.5-6.5 Gy, while irradiation up to a dose of 6.5-11.5 Gy poses a cataract risk of 66% within 4 years [12]. Historical data from Emami et al. [13] indicated a 5% risk of cataracts in 5 years (TD, 5/5) for 10 Gy lens dose, and a risk of 50% in 5 years (TD, 50/5) for a dose of 18 Gy. However, other factors, such as age, type of radiation, and fraction dose, also played a significant role.
Apart from the dosimetric benefits, interventional radiotherapy allows completion of the treatment within a period of 2 weeks in comparison with EBRT, which is delivered in 6 weeks. This is a favorable option with respect to hospital logistics as well as reduced financial burden for patients.
The retrospective nature of reporting, the availability of patients with only upper eyelid lesions, and the small number of cases (n = 4), may be considered as limitations of the current report. The median follow-up of one year confines the assessment of long-term toxicity and tumor outcomes. Similarly, there was no control group of patients receiving EBRT, thus limiting comparative assumptions.