Purpose
There are 170,000-200,000 new cases of brain metastases diagnosed each year, and 20-40% of cancer patients will develop brain metastases [1,2]. Brain metastases are especially important in the context of more effective cytotoxic, biologic, and immunologic systemic therapy, which have afforded patients longer intervals prior to developing brain metastases in passing years. This makes surveillance and management of intracranial disease increasingly important. Prognosis of patients with brain metastases are highly variable, based on the primary tumor and associated prognostic factors. Using the graded prognostic assessment (GPA) index, which divides patients into 4 tiers based on various clinical prognostic factors, median overall survival can range from 2.79 to 25.30 months [3].
The clinical management of single metastases with craniotomy and/or stereotactic radiation is well established. Level 1 evidence supports the use of stereotactic radiosurgery (SRS) alone, whole brain radiation therapy (WBRT) alone, or surgery in combination with SRS or WBRT in patients with single or multiple brain metastases (MBM) [4]. Choosing an appropriate treatment for a patient with brain metastases is quite personalized and requires close collaboration between neurosurgeons, radiation oncologists, and oncologists, in an effort to maximize and balance both survival and quality of life.
Despite its many benefits, brachytherapy is a relatively uncommon modality for the treatment of brain metastases. This treatment technique involves the implantation of radioactive isotopes at the time of tumor resection for brain metastases. Since brain metastases tend to occur relatively superficially in the brain, often in the grey-white matter interface, and are frequently surgically resected, patients with brain metastases may be ideal candidates for brachytherapy. Through this technique, one can deliver a highly conformal dose of radiation, with a rapid dose fall-off and the ability to spare surrounding normal brain tissue. The American College of Radiology (ACR) appropriateness criteria for brain metastases describes that despite similar control rates to radiosurgery, brachytherapy is rarely used because it is an invasive procedure requiring hospitalization [5]. Other reasons that may limit the usage of brachytherapy in the management of brain metastases is a rate of radiation necrosis, absence of neurosurgeons’ or radiation oncologists’ experience, and a relative lack of published data on treatment outcomes, comparing to other modalities for brain metastases.
Brachytherapy for brain tumors was first used as early as 1936, by Dr. W.O. Lodge, who implanted radon seeds in the brain of a patient who was suffering from a pituitary mass that had induced amenorrhea and vision loss [6]. The implant shrunk the tumor and restored the patients’ vision rapidly. Since then, 125I became the most frequently used brachytherapy isotope in the treatment of brain tumors, with the first treatment of brain metastases using brachytherapy in 1979 by Prados and colleagues [7]. Subsequently, other studies have been done evaluating the use of intraoperative photon radiation (photon radiosurgery – PRS) as well as other isotopes such as 131Cs [8,9,10,11,12,13]. In particular, 131Cs is a promising new isotope for the use in brachytherapy explored by Wernicke and colleagues in a series of studies on local resection followed by implantation of 131Cs seeds in patients with brain metastases [10,11,12,13].
The use of new brachytherapy modalities such as 131Cs brachytherapy may address some of the issues that have limited implementation of brachytherapy in the past. Therefore, the purpose of this paper was to provide a comprehensive summary of the literature on treatment of brain metastases with brachytherapy.
Material and methods
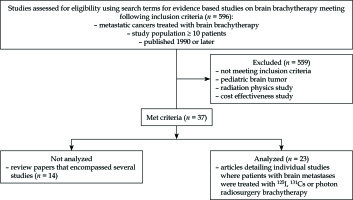
This systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [14]. A literature search of PubMed, Cochrane, and Scopus was conducted by two authors (B.C. and S.G.) using combinations of search terms and synonyms for brachytherapy, brain metastases, radiation, and published between January 1, 1990 and January 1, 2018. The search terms utilized in PubMed included: 1. “Brachytherapy” [Mesh] AND “Brain neoplasms”[Mesh]; 2. “Brachytherapy” [Mesh] AND “Brain neoplasms” [Mesh] and “Neoplasm metastasis” [Mesh]; 3. “Brachytherapy” [Mesh] and “Brain” [Mesh]. The search terms utilized in Scopus were “Brachytherapy” AND “brain” AND “secondary OR metastases OR metastasis” AND NOT “DBCOLL (medl)”. The search terms utilized in Cochrane were as follows: #1: “Brachytherapy [Mesh]”; #2: “#1 and brain”; #3: “Brachytherapy and brain and (secondary or metastases or metastasis,” #4, “#2, or #3”. In PubMed, Scopus, and Cochrane, we also utilized search terms “iridium radioisotopes” AND “intracranial neoplasm” to assess studies utilizing the 192Ir isotope. Additional manual searches in reference lists of the relevant articles were also conducted. Studies in non-English languages, duplicate articles, or studies involving animals were excluded. Papers were identified (n = 596), from which titles and abstracts were examined to eliminate studies without evidence-based data such as case reports, dosimetry studies, cost-effectiveness studies, comments/responses, reviews, stand-alone abstracts, and studies of primary brain tumors and of pediatric brain tumors. All remaining articles were screened carefully; clinical trials, large observational studies, and studies focusing on brachytherapy in patients with brain metastases received priority in the selection process. Bibliographies of these studies were searched for other relevant studies. Initially, 37 articles were identified, and review articles were excluded (n = 14). Of these, the most relevant 23 articles were selected for inclusion (Figure 1).
The resulting papers were reviewed by a multi-disciplinary team composed of medical physicists, neurosurgeons, and radiation oncologists. Critical issues were identified, and key findings from the current literature were summarized in this report. In particular, the clinical characteristics of patients used in the studies, and treatment factors such as radiation isotope (Table 1), radiation dose, and implant volume were recorded from each of the studies [15,16]. Outcome variables such as local control, rate of distant recurrence, overall survival, and treatment toxicity were also tabulated and reported. Definitions for local control and distant recurrence were tabulated as per definitions provided in individual papers. However, in general, local control refers to restriction of disease to the area immediately surrounding the resection cavity, while distant recurrence defines disease recurring or progressing outside the immediate area of the resection cavity. A notable exception included studies by Wernicke et al. and Pham et al. who reported 100% rate of local control, but some instances of regional recurrence defined as dural-based enhancement were > 5 mm from the resection cavity [10,11,12,13]. Summative assessments of treatment efficacy and toxicity were completed based on radioisotopes and brachytherapy techniques used in various studies. A statistical meta-analysis was not attempted due heterogeneity of studies and brachytherapy treatment techniques.
Table 1
Isotopes used in studies evaluating brachytherapy in treatment of brain metastases
| Isotope | Number of studies | Total # of patients of studies | mEV | t1/2 (days) | Half value layer (mm Pb) | Source |
|---|---|---|---|---|---|---|
| 125I [8,15,17,18,19,20, 21,22,23,25,26,27,28, 29,30,31,32] | 16 | 728 | .0272-.0317 | 59.4 days | 0.028 | Neutron capture of 124Xe → 125Xe → 125I (via electron capture) |
| 131Cs [10,11,12,13,16] | 4 | 79 (two studies used same 24 pts) | .0295-.0342 | 9.7 days | Neutron activation of 130Ba → 131Ba → 131Cs or nuclear reaction of 133Cs → 131Ba → 131Cs | |
| Photons [8,9] | 2 | 78 | .01 to .02 | 10^18 yrs | 1 | Delivery of electron beam of 40 µA through deflection chamber, rigid probe, and then thin gold foil (0.5 µm) producing photons with energy 10-20 kEv |
Results
Iodine-125
In the literature, most data on treatment of brain metastases with brachytherapy implement the use of 125I isotope [8,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The largest studies performed in this area include those by Raleigh et al., Ostertag et al., Petr et al., and Ruge et al. [21,22,23,29,30,31]. Raleigh et al. conducted a retrospective review featuring 95 patients with 105 brain metastases, treated between 1997 and 2013 with permanent implants, to assess treatment options for patients with recurrent or large brain metastases (Table 2). In regards to location, 32 tumors were located in the frontal lobe, 26 in the parietal lobe, 17 in the occipital lobe, 13 in the cerebellum, 94 in the cerebral/cerebellar convexity, 20 in the periventricular region, and 20 in the lobar tip. Primary tumors included 36 lung carcinomas, 26 melanomas, 22 breast tumors, and 11 tumors in other categories (Table 3). All patients received MRI, followed by a craniotomy with resection of their tumor, and implantation of permanent 125I seeds in the resection cavity. Median number of seeds implanted per cavity was 28, and median radioactivity per seed was 0.28 mCi. They reported 90% of crude local control rate and distant recurrence rate of 43% at median follow-up of 4.4 months (Table 4). Median overall survival was 12 months, and median Karnofsky Performance Score (KPS) was 80 (range, 50-90 months) (Table 5). Their overall risk of necrosis was 15% (p < 0.001), with notable increase in patients with a history of prior SRS (p < 0.05) (Table 6). Based on their results, they concluded that 125I seed brachytherapy was an effective strategy for local control of brain metastases. They also noted that volumetric parameters (e.g. metastasis or cavity volume, or rate of cavity remodeling) did not influence odds of radiation necrosis or local control. Ostertag et al. performed a study on utilization of temporary 125I in three groups: group A (38 cases) and B (40 cases) included patients with new brain metastases, and group C (21 cases) consisted of patients with recurrent brain metastases. In regards to location, 56 tumors were located in the cerebral hemispheres, 14 tumors were situated in the basal nuclei, 5 in the midbrain, 2 in the pons, and 6 tumors were located in the cerebellum. Primary tumors included 31 bronchial carcinomas, 21 hypernephromas, 18 melanomas, 18 GI tumors, 8 breast tumors, 3 uterine/ovarian tumors, two thyroid tumors, and two of unknown primaries. A radiation dose of 60 Gy was delivered at a dose rate of 7.2 cGy/h. Group A was treated with brachytherapy with adjuvant RT, while groups B and C were treated with brachytherapy alone. At median follow-up of 3 months, they reported 100% of local control rate, however with 48% of distant recurrence (outside the resection cavity) rate (Table 4). The median overall survival was 17 months for group A, 15 months for group B, and 6 months for group C (Table 5). KPS was stable or improved in 79% of patients, and there were no cases of radiation necrosis. The only reported post-operative complication was transient hemiparesis in 2% of patients (2 patients in total) (Table 6). Their work showed that high rates of local control and KPS were possible with the use of the 125I isotope for brachytherapy, even though the recurrence of disease at other brain sites remained a concern. Unfortunately, the prognosis of recurrent brain metastases was noticeably worse than that of new brain metastases, as indicated by significantly lower median OS in group C [29].
Table 2
Temporary vs. permanent implants and local brain control vs. distant brain control defs
| Study, year | # of patients | T or P | Local brain control def | Distant brain control def | # of local; # of distant recurrences | Comments |
|---|---|---|---|---|---|---|
| Alesch et al., 1995 [17] | 20 | P | “Only one patient in our series developed a local recurrence” | “One patient developed metastasis on the contralateral side after having received radiotherapy (…). Only one patient who developed a metastasis in the central region” | 1; 1 | |
| Bernstein et al., 1995 [25] | 10 | T | “Five died of recurrence in the brain at 20, 39, 52, 103, and 143 weeks post-implant; one of these recurrences was at a site distant from the brachytherapy site” | “Five died of recurrence in the brain at 20, 39, 52, 103, and 143 weeks post-implant; one of these recurrences was at a site distant from the brachytherapy site” | 4; 1 | |
| Bogart et al., 1999 [26] | 15 | P | “Local brain failure” per Venn diagram | “Distant brain failure” and “Lung/systemic failure” per Venn diagram | 3; 10 | |
| Curry et al., 2005 [9] | 60 | T | “Enlargement on follow-up MR images” | N/A | 11; 0 | |
| Dagnew et al., 2007 [27] | 26 | P | “Stable or absent contrast enhancement with the patient receiving stable or decreasing doses of steroids” | “Ten patients (38%) suffered new distant metastases within the brain: three presented with single lesions and seven had multiple lesions” | 1; 10 | |
| Huang et al., 2009 [28] | 40 | P | “No recurrent lesions at resection cavity” | “New brain metastases” | 35; 12 | |
| McDermott et al., 1996 [8] – San Francisco | 30 | P | N/A | |||
| McDermott et al., 1996 [8] – MGH/PRS | 18 | T | “Reduction or stabilization of tumor size was accepted as evidence of local control” | “At the time of this report, 13 patients have died: 12 from systemic disease and 1 from a distant CNS recurrence” | 3; 13 | |
| Ostertag et al., 1995 [29] | 93 | T | “Proliferation was controlled in every case” | “A total of 43 patients (48%) in the irradiated group died from progressive dissemination of cerebral metastases. Forty-seven patients (52%), on the other hand, died from uncontrollable growth of the primary tumor” | 0; 90 | Only 3 patients did not experience dissemination of disease |
| Petr et al., 2009 [30] | 72 | P | “Stable or absent contrast enhancement with patient receiving stable or decreasing doses of steroids” | “Twenty-three patients (32%) developed new distant metastases within the brain. Five patients developed recurrences within 3 months of initial resection that were deemed synchronous metastases with the initially resected lesions. Eighteen patients had metachronous metastases with recurrences that occurred more than 3 months after initial resection” | 5; 35 | |
| Pham et al., 2016 [12] | 24 | P | No local recurrence within 5 mm of the resection cavity | “There was one patient with regional recurrence (5 mm from the resection cavity), which was subsequently treated with SRS. All patients eventually failed distantly with median distant metastases FFP of 7.6 months (95% CI: 41.1 months, upper limited not estimated)” | 0; 24 (1 regional, 24 distant) | For this study (Pham et al.) as well as those by Wernicke et al., regional recurrence is grouped with distant recurrence as authors specify 100% of local freedom from progression in most studies. Recurrences defined as regional and those defined as distant by authors are specified |
| Raleigh et al., 2017 [31] | 95 | P | “Follow-up, local freedom from progression (LFFP) (defined as tumor recurrence within or immediately adjacent to the brachytherapy cavity), freedom from progression (defined as tumor progression at any site), freedom from necrosis (FFN), and OS were measured from the date of resection, estimated using the Kaplan-Meier method, and compared with the results of log-rank tests” | “By extrapolation: any tumor progression in the brain not “within or immediately adjacent to the resection cavity” | 10; 41 | |
| Rogers et al., 2006 [32] | 54 | T/P | “New or increased contrast enhancement within the resection cavity” | “New or increased contrast enhancement outside the resection cavity” | 9; 24 | 6 devices were not explanted after completion of the brachytherapy |
| Romagna et al., 2016 [18] | 43 | T | McDonald criteria for “In-field” and distant brain failure. Per that paper, failure – “increasing tumor size, new areas of tumor, or unequivocal neurologic deterioration” | “Distant failure” as opposed to “in-field brain failure” | 4; 15 | |
| Schulder et al., 1997 [19] | 13 | P | “Local control was defined as the absence of tumor on CT or MRI scan” | “New sites of metastatic disease to the central nervous system” | 2; 7 | “Local control was defined as the absence of tumor on CT or MRI scan” |
| Teixeira et al., 2003 [20] | 23 | T/P | N/A | |||
| Ruge et al., 2011 (Strahlenther Onkol) [23] | 77 | P | “Assessment of local tumor response on magnetic resonance imaging (MRI) scans used the MacDonald criteria [11]. The definition of complete remission, however, had to be modified for patients receiving SBT due to the frequently observed residual traces of contrast enhancement surrounding the implanted seeds resulting from treatment-induced local blood-brain barrier disruption. Local relapse was defined as a new enhancing lesion appearing in exactly the same site as the treated metastasis after complete response, or through histological confirmation by stereotactic biopsy after (re)growth of a previous partial response, or stable disease” | “Distant intracranial relapse” | 4; 36 | |
| Ruge et al., 2011 (J Neurooncol) [21] | 27 | P | “Modified version of McDonald et al. criteria, modified to account for presence of residual traces of contrast enhancement surrounding implanted seeds” | “Cerebral tumor progression distant from the site of the treated metastasis was found in 14/18 patients with available MRI follow-up scans after a median time interval of 5.4 months (range, 1.4-32.8 months) Overall, after one year, the actuarial rate of local and distant relapse was 6.7% and 45.5%” | 1; 14 | Cerebral tumor progression distant from the site of the treated metastasis was found in 14/18 patients with available MRI follow-up scans after a median time interval of 5.4 months (range, 1.4-32.8 months). Overall, after one year, the actuarial rate of local and distant relapse was 6.7% and 45.5% |
| Ruge et al., 2011 (J Neurosurg) [22] | 90 | P | “Modified version of McDonald et al. criteria” | “Distant relapse was defined as the appearance of a new enhancing lesion at a site other than the original tumor. One year actuarial rate of distant relapse” | 5; 42 | Calculated from actuarial control rate (.464 and .054* 90) |
| Wernicke et al. 2014 [10] | 24 | P | “Absence of new nodular contrast enhancement < 5 mm from the resection cavity” | “There was one case of regional recurrence, which yielded a 1-year regional resection cavity FFP of 93.8% (95% CI: 63.2%, 99.1%). This case of regional recurrence was evident 7 months post-implant and was leptomeningeal in origin. This patient was subsequently treated with SRS and is still alive at the time of analysis. There were 12 cases of distant metastases, which yielded a median distant metastases FFP of 7.6 months (95% CI: 4.1 months, upper limit not estimated) and a 1-year distant metastases FFP of 48.4% (95% CI: 26.3%, 67.4%)” | 0; 13 (1 regional, 12 distant) | Absence of new nodular contrast enhancement < 5 mm from the resection cavity |
| Wernicke et al., 2017 (Int J Radiat Oncol Biol Phys) [13] | 42 | P | “Absence of new nodular contrast enhancement < 5 mm from the resection cavity” | “Regional failure was defined as dural-based enhancement > 5 mm from the resection cavity, because such recurrences could have resulted from surgical intervention, and all other failures 5 to 20 mm from the cavity. Distant FFP was defined as the absence of new enhancement elsewhere in the brain” | 0; 22 (3 regional, 19 distant) | |
| Wernicke et al., 2017 (J Neurosurg) [11] | 13 | P | “Local failure defined as new nodular contrast enhancement ≤ 5 mm from the resection cavity. Regional failure was defined as new or increased contrast enhancement > 5 mm from the resection cavity. Note, while authors use FFP, we calculated local, distant or regional failure as a fraction of total brain metastases, at 1 yr, for sake of consistency with other studies in this analysis” | “Regional failure was defined as new or increased contrast enhancement > 5 mm from the resection” | 1; 5 (2 regional, 3 distant) | |
| Zamorano et al., 1992 [24] | 18 | T/P | N/A | N/A | N/A | 16 temporary, 2 permanent implants |
Table 3
Tumor characteristics in studies evaluating brachytherapy in treatment of brain metastases
| Study, year | # of patients | Primary tumor | Sites in brain | Implant | Median tumor volume |
|---|---|---|---|---|---|
| Alesch et al., 1995 [17] | 20 | Lung (8), breast (3), colon (3), larynx (2), kidney (1), thyroid (1) | Frontal (8), parietal (5), temporal (3), central (1), basal ganglia (2), pontine (1) | 125I | 4.2 |
| Bernstein et al., 1995 [25] | 10 | Lung adenocarcinoma (9), breast adenocarcinoma (1) | Cerebral hemispheres (9), cerebellar (1) | 125I | 36.4* |
| Bogart et al., 1999 [26] | 15 | Lung (15; NSCLC) | Frontal (5), parietal (5), occipital (4) temporal (1) | 125I | 8.2 |
| Curry et al., 2005 [9] | 60 | Lung (33), melanoma (15), renal cell (5) breast (2), esophageal (2), colon (1), and Merkle cell (1) malignant fibrous histiocytoma (1) | Frontal (29), frontoparietal (4), parietal (13), temporal (17), temporoparietal (2), parieto-occipital (1), occipital (4), basal ganglia (1), cerebellar (1) | PRS | 7.8* |
| Dagnew et al., 2007 [27] | 26 | Lung (12), melanoma (4) colon (3), breast (2), renal (1), cervix (1), prostate (1), ovarian (1), unknown (1) | 125I | 14.1 | |
| Huang et al. 2009 [28] | 40 | Melanoma (8), lung (7), breast (2), other (2)** | Frontal (11), parietal (7), frontoparietal (4), temporal (11), occipital (4), temporo-occipital (1), occipitoparietal (1), cerebellar (5) | 125I | 17.2 |
| McDermott et al., 1996 [8] – San Francisco | 30 | Adenocarcinoma (15), melanoma (8), angiosarcoma (1), rhabdomyosarcoma (1), Ewing’s sarcoma, small cell carcinoma (1), endometrial carcinoma (1), undifferentiated sarcoma (1), unknown (1) | N/A | 125I | 20.6* |
| McDermott et al., 1996 [8] – MGH/ PRS | 18 | Histology not specified; all lesions were supratentorial | N/A | PRS | 4.9 |
| Ostertag et al., 1995 [29] | 93 | Bronchial carcinoma (NSCLC; 31), hypernephroma (21), melanoma (18), gastrointestinal (18), breast (8), uterus/ovary (3), thyroid (2), unknown (2) | Cerebral hemispheres (66), basal nuclei (14), midbrain (5), pons (2), cerebellar (6) | 125I | 16.5 |
| Petr et al., 2009 [30] | 72 | Lung (38; NSCLC), breast (9), colon (6), melanoma (5), ovarian (3), renal (3), prostate (1), cervical (1), bladder (1), unknown (4) | Supratentorial (55), infratentorial (17) | 125I | 14.1 |
| Pham et al., 2016 [12] | 24 | Lung (16), breast (2), kidney (2), melanoma (2), colon (1), cervix (1) | Frontal (10), parietal (7), temporal (1), occipital (2), cerebellar (4) | 131Cs | 10.3 |
| Raleigh et al., 2017 [31] | 95 | Lung (36), melanoma (26), breast (22), other (11) | Frontal (32), parietal (17), temporal (26), occipital (17), cerebellum (13), cerebral/cerebellar convexity (94), periventricular (20), lobar tip (20) | 125I | 13.5 |
| Rogers et al., 2006 [32] | 54 | Lung (29), gastrointestinal (7), melanoma (7), renal (3), other (8) | Frontal (15), parietal (12), temporal (6), occipital (7), other (14) | 125I | 14.1 |
| Romagna et al., 2016 [18] | 43 | Lung (17; 11 NSCLC, 2 SCLC, 4 other), skin (5), gastrointestinal (3),kidney (3), uterus (1), ovary (1), musculoskeletal (1), prostate (1) | N/A | 125I | 2.6 |
| Schulder et al., 1997 [19] | 13 | Lung (4; NSCLC), breast (3), germ cell (3: testicle 2, mediastinum 1), melanoma (2), renal (1) | Frontal (4), parietal (4), temporal (1), occipital (1) | 125I | 14.1 |
| Teixeira et al., 2003 [20] | 23 | Lung (7), breast (4), other/unknown/undifferentiated (5) | Including patients in study with primary brain tumors (NOT just metastases) 63% of cases were in cerebral hemispheres, 21.8% in deep structures, 13.8% in brainstem | 125I | 38.3 |
| Ruge et al., 2011 (Strahlenther Onkol) [23] | 77 | Lung (20; NSCLC), breast (16), kidney (10), melanoma (7), colon (6), other (12), unknown (6) | Cerebral hemispheres (42), pons (10), basal ganglia/diencephalon (15), cerebellar (8), other (2) | 125I | |
| Ruge et al., 2011 (J Neurooncol) [21] | 27 | Breast (11), lung (5; NSCLC) melanoma (3), colorectal (3), kidney (1), esophagus (1), other (2), unknown (1) | N/A | 125I | |
| Ruge et al., 2011 (J Neurosurg) [22] | 90 | Lung (27; NSCLC), breast (17), kidney (12), melanoma (8), colorectal (7), other (13), unknown (6) | Cerebral hemispheres (26), pons (12), insular (6), pre/post central sulcus (19), basal ganglia/diencephalon (13), other (2) | 125I | * |
| Wernicke et al., 2014 [10] | 24 | Lung (16), breast (2), kidney (2), melanoma (2), colon (1), cervix (1) | Frontal (10), parietal (7), temporal (1), occipital (2), cerebellar (4) | 131Cs | 10.3 |
| Wernicke et al., 2017 (Int J Radiat Oncol Biol Phys) [13] | 42 | Lung (26), colon (4), breast (3), melanoma (2), uterus (2), esophagus (5), kidney (1), hepatobiliary (1), tonsillar (1) | Frontal (14), parietal (14), temporal (4), occipital (3), cerebellar (11) | 131Cs | 14.1 |
| Wernicke et al., 2017 (J Neurosurg) [11] | 13 | Lung (9), melanoma (3), breast (1), gastric (1), pancreatic (1) | Frontal (3), parietal (4), temporal (3), occipital (2), cerebellar (2), insular (1) | 131Cs | 12.8 |
| Zamorano et al., 1992 [24] | 18 | N/A | N/A | 125I | |
* Most volumes listed were calculated from tumor diameter via 4/3 ϖ (D/2)3 and represent median volume.
Exceptions: Bernstein et al., 1995 [25]: volume listed is implant volume, Curry et al., 2005 [9]: volume listed is mean treatment volume, Ruge et al., 2011 [22] (J Neurosurg): 70 patients had tumor volume < 14 cm, 20 patients had tumor volume > 14 cm; McDermott et al., 1996 [8] San Francisco: volume listed = isodose volume
Table 4
Extent of local brain control in studies evaluating brachytherapy in treatment of brain metastases
| Study, year | # of patients | Implant | Fxn with local brain control | Time used for LBC/FFP | LBC def |
|---|---|---|---|---|---|
| Alesch et al., 1995 [17] | 20 | 125I | 95% | No local progression | |
| Bernstein et al., 1995 [25] | 10 | 125I | 40% | 81 | No local recurrence |
| Bogart et al., 1999 [26] | 15 | 125I | 66% | No recurrent at or adjacent to primary site | |
| Curry et al., 2005 [9] | 60 | PRS | 81% | 6 | Demonstrated stabilization or reduction in tumor size on MRI |
| Dagnew et al., 2007 [27] | 26 | 125I | 96% | 12 | Stable or absent contrast enhancement with patient receiving stable or decreasing doses of steroids |
| Huang et al., 2009 [28] | 40 | 125I | 88% | 12 | No recurrent lesions at resection cavity |
| McDermott et al., 1996 [8] – San Francisco | 30 | 125I | 14.5-49 | N/A | |
| McDermott et al., 1996 [8] – MGH/PRS | 18 | PRS | 83% | 1.5-24 | Reduction or stabilization of tumor size was accepted as evidence of local control |
| Ostertag et al., 1995 [29] | 93 | 125I | 100% | 3 | Proliferation was controlled in every case |
| Petr et al., 2009 [30] | 72 | 125I | 93% | Stable or absent contrast enhancement with patient receiving stable or decreasing doses of steroids | |
| Pham et al., 2016 [12] | 24 | 131Cs | 100% | 19.3 | No local recurrence within 5 mm of the resection cavity |
| Raleigh et al., 2017 [31] | 95 | 125I | 90% | 14.4 | Local freedom from progression (i.e. no tumor recurrence within or immediately adjacent to the brachytherapy cavity) |
| Rogers et al., 2006 [32] | 54 | 125I | 83% | 12 | New or increased contrast enhancement within the resection cavity |
| Romagna et al., 2016 [18] | 43 | 125I | 91% | 12 | McDonald criteria for “in-field” and distant brain failure. Per that paper, failure = “increasing tumor size, new areas of tumor, or unequivocal neurologic deterioration” |
| Schulder et al., 1997 [19] | 13 | 125I | 69% | Local control was defined as the absence of tumor on CT or MRI scan | |
| Teixeira et al., 2003 [20] | 23 | 125I | N/A | ||
| Ruge et al., 2011 (Strahlenther Onkol) [23] | 77 | 125I | 95% | 12 | Assessment of local tumor response on magnetic resonance imaging (MRI) scans used the MacDonald criteria [11]. The definition of complete remission, however, had to be modified for patients receiving SBT due to the frequently observed residual traces of contrast enhancement surrounding the implanted seeds resulting from treatment-induced local blood-brain barrier disruption. Local relapse was defined as a new enhancing lesion appearing in exactly the same site as the treated metastasis after complete response, or through histological confirmation by stereotactic biopsy after (re)growth of a previous partial response, or stable disease |
| Ruge et al., 2011 (J Neurooncol) [21] | 27 | 125I | 92% | 12 | Modified version of McDonald et al. criteria, modified to account for presence of residual traces of contrast enhancement surrounding implanted seeds |
| Ruge et al., 2011 (J Neurosurg) [22] | 90 | 125I | 98% | 12 | Modified version of McDonald et al. criteria |
| Wernicke et al., 2014 [10] | 24 | 131Cs | 100% | 12 | Absence of new nodular contrast enhancement < 5 mm from the resection cavity |
| Wernicke et al., 2017 (Int J Radiat Oncol Biol Phys) [13] | 42 | 131Cs | 100% | 12 | Absence of new nodular contrast enhancement < 5 mm from the resection cavity |
| Wernicke et al., 2017 (J Neurosurg) [11] | 13 | 131Cs | 93% | 12 | Local failure defined as new nodular contrast enhancement ≤ 5 mm from the resection cavity. Regional failure was defined as new or increased contrast enhancement > 5 mm from the resection cavity. Note, while authors use FFP, we calculated local, distant or regional failure as a fraction of total brain metastases, at 1 yr, for sake of consistency with other studies in this analysis |
| Zamorano et al., 1992 [24] | 18 | 125I | N/A | N/A | N/A |
Table 5
Survival rates in studies evaluating brachytherapy in treatment of brain metastases
| Study, year | # of patients | Implant | 12 months survival rate | Median overall survival (months) |
|---|---|---|---|---|
| Alesch et al., 1995 [17] | 20 | 125I | ||
| Bernstein et al., 1995 [25] | 10 | 125I | 50% | 11.5 |
| Bogart et al., 1999 [26] | 15 | 125I | 13% | 14 |
| Curry et al., 2005 [9] | 60 | PRS | 34% | 8 |
| Dagnew et al., 2007 [27] | 26 | 125I | 72% | 17.8 |
| Huang et al., 2009 [28] | 40 | 125I | 48% | 11.3 |
| McDermott et al., 1996 [8] – San Francisco | 30 | 125I | 55% | 14.7 |
| McDermott et al., 1996 [8] – MGH/PRS | 18 | PRS | ||
| Ostertag et al., 1995 [29] | 93 | 125I | Lung – 42%, hypernephroma – 66%, melanoma – 50% | 17 (group A), 15 (group B), 6 (group C) |
| Petr et al., 2009 [30] | 72 | 125I | 55% | 14 |
| Pham et al., 2016 [12] | 24 | 131Cs | ||
| Raleigh et al., 2017 [31] | 95 | 125I | 12 | |
| Rogers et al., 2006 [32] | 54 | 125I | 40% | 40 |
| Romagna et al., 2016 [18] | 43 | 125I | 21.2 | |
| Schulder et al., 1997 [19] | 13 | 125I | 38% | 9 |
| Teixeira et al., 2003 [20] | 23 | 125I | > 40% | 10 |
| Ruge et al., 2011 (Strahlenther Onkol) [23] | 77 | 125I | 8 | |
| Ruge et al., 2011 (J Neurooncol) [21] | 27 | 125I | 14.8 | |
| Ruge et al., 2011 (J Neurosurg) [22] | 90 | 125I | 8.5 | |
| Wernicke et al., 2014 [10] | 24 | 131Cs | 50% | 9.9 |
| Wernicke et al., 2017 (Int J Radiat Oncol Biol Phys) [13] | 42 | 131Cs | 58% | 15.1 |
| Wernicke et al., 2017 (J Neurosurg) [11] | 13 | 131Cs | 25% | 7 |
| Zamorano et al., 1992 [24] | 18 | 125I | 44% | 11 |
Table 6
Treatment complications in studies evaluating brachytherapy in treatment of brain metastases
| Study, year | # of patients | Implant | Necrosis | Fxn other acute post-op complication | Comments on acute post-op complication | Fxn with other complication caused by implant | Comment on other complication |
|---|---|---|---|---|---|---|---|
| Alesch et al., 1995 [17] | 20 | 125I | 0% | 0% | N/A | 0% | N/A |
| Bernstein et al., 1995 [25] | 10 | 125I | 30% | 20% | Both had suspected pulmonary embolus | 20% | Both had permanent worsening of pre-existing motor weakness |
| Bogart et al., 1999 [26] | 15 | 125I | 0% | 7% | 1 fungal infection | 0% | N/A |
| Curry et al., 2005 [9] | 60 | PRS | 5% | 15% | Post-op seizures (4), cerebral edema (3), hemorrhage (2), also not included – radiation necrosis = 3 | N/A | N/A |
| Dagnew et al., 2007 [27] | 26 | 125I | 3% | N/A | N/A | N/A | N/A |
| Huang et al., 2009 [28] | 40 | 125I | 23% | N/A | N/A | 2.5% | 1 patient had mild permanent progressive speech hesitancy |
| McDermott et al., 1996 [8] – San Francisco | 30 | 125I | 10% | N/A | N/A | N/A | N/A |
| McDermott et al., 1996 [8] – MGH/PRS | 18 | PRS | N/A | 22% | Transient new neurologic deficits (2), partial seizures (2) | 0% | N/A |
| Ostertag et al., 1995 [29] | 93 | 125I | 0% | 2% | Transient hemiparesis (2) | N/A | N/A |
| Petr et al., 2009 [30] | 72 | 125I | 6% | 8% | 7% had thromboembolic events, 1% had a post-op infection | N/A | N/A |
| Pham et al., 2016 [12] | 24 | 131Cs | 0% | N/A | N/A | N/A | N/A |
| Raleigh et al., 2017 [31] | 95 | 125I | 15% | 6% | Wound complication | N/A | N/A |
| Rogers et al., 2006 [32] | 54 | 125I | 7% | 13% | 1 each of grade 3 CSF leak, headache, hemiplegia, hydrocephalus, infection, intracranial hemorrhage and grade 2 seizure | N/A | N/A |
| Romagna et al., 2016 [18] | 43 | 125I | 0% | N/A | N/A | N/A | N/A |
| Schulder et al., 1997 [19] | 13 | 125I | 15% | 15% | Intracerebral hematoma/PE in one, and ARDS in another | 15% (1 bone flap infection, 1 CSF leak, both treated w/o further sequalae) | N/A |
| Teixeira et al., 2003 [20] | 23 | 125I | N/A | 5% | 7/138; 5 patients had infection – 3 with skin infection and 2 with osteomyelitis and 2 patients had incisional CSF leakage | N/A | N/A |
| Ruge et al., 2011 (Strahlenther Onkol) [23] | 77 | 125I | 0% | N/A | N/A | N/A | N/A |
| Ruge et al., 2011 (J Neurooncol) [21] | 27 | 125I | 0% | 7% | 1 patient developed wound infection, 1 patient developed transient aphasia | N/A | N/A |
| Ruge et al., 2011 (J Neurosurg) [22] | 90 | 125I | 4% | Acute renal failure post-surgery (1), superficial wound infection (2), CSF fistula (1) | N/A | N/A | |
| Wernicke et al., 2014 [10] | 24 | 131Cs | 0% | 13% | CSF leak (1), seizure (1), infection (1) | N/A | N/A |
| Wernicke et al., 2017 (Int J Radiat Oncol Biol Phys) [13] | 42 | 131Cs | 0% | 26% | 11 – seizures (6, in patients w/no hx of seizures), superficial wound infections (3), CSF leak (1 patient who already developed superficial wound infection) , intracranial infection (1), 1 who developed brachytherapy seed migration | N/A | N/A |
| Wernicke et al., 2017 (J Neurosurg) [11] | 13 | 131Cs | 0% | 46% | 3 infections, 1 seizures and 1 pseudo-meningocele | N/A | N/A |
| Zamorano et al., 1992 [24] | 18 | 125I | N/A | N/A | Worsened KPS after tx | 33% (5/16 temporary, and 1/2 permanent implants) | Remaining 67% (11/16 temporary and 1/2 permanent) had stable or improved KPS |
Petr et al. studied the use of surgical resection and permanent 125I seeds for treatment of newly diagnosed brain single metastasis in 72 patients, between 1997 and 2007. Of the tumors treated, 66 were located in the cerebral hemispheres, 14 in the basal nuclei, 5 in the midbrain, 2 tumors were situated in the pons, and 6 in the cerebellum. Primary tumor sites included 38 lung (non-small cell lung cancer specifically), 9 breast, 6 colon, 5 melanoma, 3 ovarian, 3 renal, 1 prostate, 1 cervical, 1 bladder, and 4 of unknown malignancies (Table 3). A radiation dose of 150 Gy was delivered, with seed activity ranging from 4.04 to 40.38 mCi. They reported 93% of local control, distant brain failures in 32% of patients, and median OS of 14 months (Tables 4 and 5). The treatment was tolerable, and 100% of patients had stable or improved KPS. However, there was a 6% rate of radiation necrosis and 8% rate of other post-operative complications (Table 6). They demonstrated local control rates that compare favorably to WBRT while sparing patients’ functional deterioration often associated with receiving WBRT, as indicated by stable or improved KPS in patients receiving brachytherapy. However, rates of distant recurrence were higher than in studies utilizing upfront WBRT [30].
Ruge and colleagues conducted a series of studies on 125I brachytherapy. The first of their studies compared permanent interstitial 125I brachytherapy (77 patients) with stereotactic radiosurgery (142 patients) for treatment of de-novo singular brain metastases. Of these patients, 42 patients had disease in the cerebral hemispheres, 10 had tumors in the pons, 15 in the basal ganglia/diencephalon, 8 had disease in the cerebellum, and 2 had tumors located elsewhere. Primary sites included 20 lung tumors, 16 breast tumors, 3 melanomas, 3 colorectal tumors, 1 kidney tumor, 1 esophageal tumor, two tumors listed as other, and 1 of unknown primary (Table 3). Ruge et al. found that brachytherapy was overall comparable to SRS, with greater rates of local control vs. SRS, with 94.6% vs. 92.8%, respectively, similar rates of distant control, with 53.6% vs. 57.6%, respectively, and comparable median survival, with 8.0 vs. 8.1 months, respectively (Tables 4 and 5) [23]. The aim of their second study was to distinguish radiation-induced tumor changes and progression of disease in 30 patients with previously irradiated, locally recurrent brain metastases assessed with stereotactic biopsy. Twenty-seven of these patients had no signs of radiation necrosis on biopsy, and received 50 Gy of permanent 125I brachytherapy for 42 days (Table 6). Primary tumors among treated patients included 11 breast, 5 lung (non-small cell lung cancer), 3 melanoma, 3 colorectal, 1 kidney, 1 esophagus, two other, and one of unknown origin (Table 3). Their rates of local and distant control were 92.3% and 54.5%, respectively, with median overall survival of 14.8 months (Tables 4 and 5). Furthermore, 94% of patients displayed stable or improved KPS at 3 months follow-up. No patients experienced radionecrosis, and 6.6% of patients experienced post-operative complications, including one with a wound infection and one with transient aphasia (Table 6) [21]. Their third study included 90 patients with singular brain metastases treated with stereotactic permanent 125I brachytherapy. Of these, 26 patients had primary tumors of the lung, 17 of the breast, 12 of the kidney, 8 melanomas, 7 colorectal tumors, 13 tumors of other primary site, and 6 tumors of unknown primary site. Locations of these tumors included 26 tumors in the cerebral hemispheres, 12 tumors in the pons, 6 insular tumors, 19 pre/post-central sulcus, 13 basal ganglia/diencephalon, and 2 in another locations (Table 3). They found that brachytherapy compared well to other local therapies, namely surgery and SRS, with rates of local disease control of 94.6%, distant disease control of 53.6%, and median overall survival of 8.5 months (Tables 4 and 5). Of note, only 4.4% of patients experienced post-operative complications, including acute renal failure post-surgery (1 case), superficial wound infection (2 cases), and CSF fistula (1 case) (Table 6) [22].
These large studies evaluating 125I brachytherapy demonstrate that excellent rates of local control, good rates of overall survival, and improvements in quality of life were possible to achieve. However, rates of regional recurrence, rates of radiation necrosis, and other post-operative complications needed an improvement.
Photon radiosurgery
In addition to 125I brachytherapy, some studies have examined the use of photon radiosurgery (PRS) as a modality of brachytherapy for brain metastases [8,9]. The photon radiosurgery device (Photoelectron Corp, Lexington, MA, United States) consist of a miniaturized X-ray source at the end of a small minimally invasive interstitial probe. Electrons from a small battery-powered thermionic gun are accelerated to a final energy of up to 40 keV and directed along a tube to a thin Au target, where the beam size is approximately 0.3 mm. X-ray output, which is nearly isotropic, consists of a bremsstrahlung spectrum and several lines between 7 and 14 keV [33]. In a study of McDermott et al., PRS doses ranging from 10-26 Gy were used with WBRT for treatment of 18 patients with supratentorial brain metastases (Table 3). Local control rates of 83% was achieved, with regional recurrence in only 1 of 18 patients (5.6%) and transient acute post-op complications in 22% of patients (Tables 4 and 6). Additionally, a greater control of radioresistant lesions with PRS was obtained compared to 90% of external radiosurgery [8]. Curry et al. delivered stereotactic low activity photons via a photon radiosurgery system (PRS) for treatment of 60 brain metastases. Tumor locations included frontal lobe (29 of patients), frontoparietal (4), parietal (13), temporal (17), temporoparietal (2), parieto-occipital (1), occipital (4), basal ganglia (1), and cerebellar (1 case). Primary tumor sites included 33 lung tumors, 15 melanoma, 5 renal, 2 breast, 2 esophagus, 1 colon, 1 Merkle cell, and 1 malignant fibrous astrocytoma (Table 3). Local brain control rate of 81.4% was achieved, with median OS of 8 months (Table 4 and 5). There was a radiation necrosis rate of 5% and a 15% rate of other acute post-operative complications (Table 6) [9].
Cesium-131
Most studies on 131Cs brachytherapy for treatment of brain metastases have been performed by Wernicke and colleagues including 24 patients in two studies and 42 in another research. Patients were treated with local resection, followed by implantation of permanent 131Cs seeds (Table 2) [10,11,12,13]. These studies reported 100% of local brain control, low rates of regional recurrence, and distant progression within the brain, with no cases of radiation necrosis and minimal post-operative complications (Tables 4 and 6). Their first study involved 24 patients, with disease sites including 10 frontal, 7 parietal, 4 cerebellar, 2 occipital, and 1 temporal tumor. Primary tumors consisted of 16 lung, 2 breast, 2 kidney, 2 melanoma, 1 colon, and 1 cervix cancer. They delivered an 80 Gy dose at 5mm depth from the resection cavity. With median follow-up of 12 months, they achieved 100% rate of local control, with regional recurrence rate of 6.2%, distant recurrence rate of 51.6%, and median OS of 9.9 months (Table 5). There were no cases of radiation necrosis, although complications occurred in 12.5% of patients and included a cerebrospinal fluid leak, a seizure, and an infection (Table 6) [10].
Their second study assessed the use of 131Cs brachytherapy for large tumors, defined as tumors > 2.0 cm in diameter, which historically have higher rate of radiation necrosis as well as recurrence. Stereotactic radiosurgery (SRS), which generally offers excellent local control suffers from high rates of recurrence in large tumors > 3.0 cm in diameter. In a phase 2 trial of SRS by Brennan et al., a 2-year actuarial control rate was achieved in only 40% in tumors > 3.0 cm vs. 89% in those < 3.0 cm [34,35]. A study done by Wernicke et al. included 42 patients, with 14 parietal, 14 frontal,11 cerebellar, 3 occipital, and 4 temporal metastases. Histology featured 26 lung, 4 colon, 3 breast, 2 melanoma, 2 uterine, 2 esophageal, 1 kidney, 1 hepatobiliary, and 1 tonsillar tumor (Table 3). Their disease control rates included 100% of local control rate, additionally noted a 7.1% of regional recurrence rate, distant recurrence rate of 54% at 12 months, and overall survival of 15.1 months (Tables 4 and 5). While no case of radiation necrosis was reported, complications were seen in 26% of patients, including 6 seizures in patients with no prior history of seizures, one intracranial infection, one case of brachytherapy seed migration, and superficial wound infections seen in 3 patients, one of whom also had a CSF leak (Table 6).
In addition to the aforementioned studies, Wernicke et al. conducted a research utilizing 131Cs brachytherapy as a salvage treatment, including 13 patients with recurrent brain metastases resistant to SRS and/or WBRT. Of these, 3 tumors were in the frontal lobe, 4 parietal, 2 occipital, 3 temporal, 2 cerebellar, and 1 insular. Histology included 9 lung tumors, 3 melanomas, 1 breast, 1 pancreatic, and 1 gastric tumor (Table 3). The prescription dose was 80 Gy located at 5 mm from the resection cavity surface. The 1-year local control rate was 93.3%, with 13.3% of regional recurrence and 20% of distant recurrence (Table 4). In a median OS of 7 months, radiation necrosis rate was 0%; however, a rate of acute post-operative complications occurred in 46% of patients (Tables 5 and 6). This was attributed to poor general condition of patients and small size of investigated cohort [11].
Studies on standard of care therapies for brain metastases, e.g. WBRT and SRS, have demonstrated that the treatment with these modalities may lead to an acute decline in cognitive function, as measured by FACT-BR questionnaire [36,37]. This questionnaire assesses physical, functional, and emotional well-being. Irrespective of treatment modality, radiologic control of disease was associated with decreased decline in cognitive function, as measured by the mini-mental status exam (MMSE) score [38]. A decline in scores over 3 months was 0.5 for those with well controlled disease vs. that of poorly radiologically controlled, with a decline of 6.3. The first evaluation of 131Cs brachytherapy per these indices showed a promise. Pham et al. found that 131Cs brachytherapy at least preserves quality of life in patients with brain metastases, on the basis of FACT-BR questionnaire score increase from 146.5 to 164 at 6 months post-treatment. Furthermore, an improvement in MMSE score of all patients was observed, including patients with a pretreatment MMSE score < 27 with an increase to a score of 30 [12].
Discussion
The purpose of this review was to provide a summary of the published data using brachytherapy for the treatment of brain metastases. Goals included identifying brachytherapy techniques with the most supportive data, and recognizing important questions to improve the efficacy and safety of this treatment modality.
The majority of data on treatment of brain metastases with brachytherapy uses the 125I isotope. 125I brachytherapy produces excellent rates of local control and overall survival as well as improvements in KPS score [21,29,30]. It additionally demonstrates a promise as an effective salvage therapy for recurrent brain metastases [21]. Unfortunately, this technique tends to result in high rates of radiation necrosis, and post-operative complications may explain why brachytherapy has not been commonly used in the treatment of brain metastases [21,30]. This is particularly important because not only can radiation necrosis be symptomatic, but even when asymptomatic, it may preclude further therapy [21]. Due to the heterogeneity of the studies and different reporting methods, conclusions regarding the rates of symptomatic versus asymptomatic radiation necrosis were not established.
As an alternate method of brachytherapy, the photon radiosurgery (form of electronic brachytherapy) device has been presented. PRS is limited by greater toxicity and rates of local control that are at best, comparable to 125I seed therapy. However, PRS is notable for excellent rates of regional control and greater control of radioresistant lesions than external radiosurgery [8]. Though PRS suffers from potentially use limiting issues of toxicity like 125I seed BT, its excellent rates of regional control may warrant further investigation in the treatment of brain metastases. The rates of radiation necrosis are comparable to 125I seed brachytherapy, with higher rates of post-operative complications [8,9]. Another major limitation of PRS is that the device used in many of the clinical studies is no longer commercially available. The field awaits the development of another intraoperative or electronic brachytherapy device specialized in intracranial applications [33].
The most recent development in brain brachytherapy is the use of the 131Cs isotope. This isotope shows promising results regarding toxicity, which did not permit brachytherapy to be commonly used for treatment of brain metastases, namely high rates of radiation necrosis and post-operative complications. Studies by Wernicke and colleagues on 131Cs seed implantation, preceded by surgical resection of tumor, are significant for no cases of radiation necrosis and limited post-operative complications related to 125I seed implantation [10,11,12,13]. These results, especially the lack of radiation necrosis in 131Cs as compared to 125I, can be partially explained by several radiobiological advantages of 131Cs over 125I. Firstly, 131Cs has a higher median energy, enabling the use of fewer seeds in a given tumor volume. In addition, it has a higher dose-rate, thereby limiting radiation exposure by allowing delivery of greater proportion of dose in a short time. 131Cs’s shorter half-life further limits the duration of patient’s exposure to radiation [11]. Relatively low radiation necrosis rates in 131Cs may also be explained by high quality of neurological technique or planning methods, as all these studies were done by Wernicke and colleagues. For instance, low seed activity combined with low radiation dose would cause minimize radiation necrosis, so the treatment was planned accordingly [10]. Studies with the use of 125I have been done by a wide variety of groups, hence the quality of technique or planning methods may not be as high.
One final reason for the lower rate of radionecrosis in the 131Cs data compared to 125I may simply be the lower biological equivalent dose delivered to normal tissue. A comparison of doses was difficult in the past because of uncertainties in estimating the equivalent prescription between the isotopes based on linear quadratic equation (LQE) and biological equivalent dose (BED) formalism. In 2014, Luo et al. published conversion factors between 125I and 131Cs prescription doses, with a resensitization correction for fast and slow growing tissues [39]. Therefore, the Petr study, which used 125I implants with a prescription dose of 150 Gy at 5 mm, and which resulted in high radionecrosis rates, would be biologically equivalent to a 131Cs equivalent dose of 110 Gy for tumor (α/β ratio of 10) and a 131Cs equivalent dose of 149 Gy for normal tissue (α/β ratio of 3) [30]. This is a biological equivalent dose that is considerably higher than the 80 Gy 131Cs dose at 5 mm that is typically prescribed today. Huang et al. used 125I with a dose of 200 Gy at 1 cm from the cavity, and also reported a high radionecrosis rate of 26% [28]. Other 125I studies, which used lower prescription doses in the range of 50-60 Gy (131Cs equivalent doses of 40-50 Gy for normal tissue) reported low rates of radionecrosis [21,22,23,29]. Lower equivalent doses used in 131Cs brachytherapy appear to result in similar local control to high-dose 125I while limiting toxicity. Therefore, radiobiologic knowledge of low-dose-rate brachytherapy is important for understanding the risk of toxicity of brain brachytherapy implants.
In addition to decreasing toxicity, 131Cs brachytherapy may improve quality of life as measured by FACT-BR questionnaire and mini-mental status exam [12]. Recent studies on 131Cs have achieved up to 100% of local control, durable regional and distant control of disease resistant to SRS and WBRT [10,11,12,13]. The ability of 131Cs brachytherapy to accomplish excellent control of disease with limited toxicities, especially compared to therapies such as SRS and WBRT, support the use of brachytherapy as a more conventional treatment for brain metastases [11]. 131Cs brachytherapy may also result in improvement in quality of life as measured by FACT-BR questionnaire and the mini-mental status exam [12].
Considering the present state of brachytherapy and all available modalities used to treat brain metastases, 131Cs brachytherapy shows a significant promise. Both 125I and 131Cs brachytherapy are notable for excellent rates of both local and regional control, with 131Cs possessing ideal radiobiological properties and with possible improvements in radiation necrosis as compared to 125I brachytherapy as well as quality of life [10,11,12,13]. This reduction of toxicity may support wider implementation of brachytherapy as a therapy for patients with brain metastases, particularly for those with large or recurrent tumors. Furthermore, it has low rates of radiation necrosis and other post-operative complications. It should be noted that there were no studies that met our eligibility criteria that utilized high-dose-rate brachytherapy with 192Ir.
Reasons that currently limit the use of brachytherapy are as follows: 1) The status of brachytherapy as an invasive procedure necessitating hospitalization; 2) The absence of radiation oncologists’ or neurosurgeons’ expertise in brachytherapy; 3) The lack of published data on treatment outcomes; 4) The increasing role of stereotactic radiosurgery, which is a minimally invasive procedure used to treat many of the same tumors that can be treated with brachytherapy. Even with these limitations, brachytherapy is well suited for treatment of brain metastases, through its ability to deliver a high-dose of radiation confined to the resection cavity, while sparing adjacent radiosensitive tissues. This precision achieved by brachytherapy results in excellent rates of local control and improved quality of life.
Conclusions
The studies examining brachytherapy in the management of brain metastases are predominantly single center studies, with inconsistencies in reporting, quality control, and choice of isotope. However, the results indicate that brachytherapy warrants further consideration in the management of brain metastases, especially in the setting of recurrent tumors after an initial course of radiation therapy. In addition, more studies must be completed to evaluate brachytherapy as a widely used and accepted method of treatment for brain metastases.