Purpose
Currently, 3D image-guided brachytherapy with radiation dose delivered with boost as a part of locally advanced cervical cancer treatment is the recommended and well-established technique, with advantages in local control [1-4]. In addition, 3D MRI-guided brachytherapy enables brachytherapy to be individually tailored according to residual tumor volume [5]. MR imaging-based planning can have a more precise target delineation compared with CT-based target contouring [6]. To cover asymmetrical and large tumors precisely and adequately, brachytherapy applicators had evolved to allow simpler use of interstitial needles. Interstitial needles, along with applicator’s internal channels, offer the potential to increase the dose coverage of tumors, which are outside of traditional pear-shaped dose distribution.
Recently, there has been a rapid development of applicators for cervical cancer brachytherapy, allowing intra-cavitary and interstitial combined approaches. For better dose coverage, these applicators have applicator pre-defined needle (APN) positions, such as Elekta’s (Elekta AB, Stockholm, Sweden), Venezia™, and Geneva applicators. Moreover, Varian Medical Systems, Inc. has also introduced equivalent applicators, i.e., Aarhus, 3D interstitial ring and FSD-style applicator sets. From those mentioned, Venezia™, Aarhus, and FSD-style (with interstitial components) applicators allow the use of needles that are not parallel to intra-uterine (IU) tandem, but are rather oblique. These directions are good examples of reaching distal parametrial tumors [7]. Although modern applicators provide many possibilities for needle location, there are still challenges in reaching large and irregular tumors only with the applicator and its APNs for interstitial needles. For such challenging clinical cases, where no APN channel at optimal place is available, it is possible to use extra FHNs inserted into the tumor independently form the applicator.
Because APN positions and angles are not limiting the location and direction of FHNs, they can be inserted at free angles, and the insertion point can be chosen without any restrictions. In addition, FHNs do not have any insertion depth restrictions other than the length of the needle. For example, with Elekta applicators and needle guiding tubes, APNs have a 5 cm depth limit from the applicator surface with the needle insertion tool.
Indications for the use of FHNs include the whole uterine corpus that needs to be treated, intra-uterine tandem that is not reaching deep enough, when tumor at the parametrium of the cervix is out of reach of APNs, or tumor spread at vaginal wall. There are differences between various applicators; therefore, indications for the use of FHNs vary according to the applicator. Figure 1 demonstrate a case, where FHNs are needed for adequate dose coverage.
Fig. 1
Example of a plan with free-hand needles (FHNs) on the left (patient right) side of the applicator ring part. Oblique APN cannot reach the tumor. Green isodose = 7 Gy. Image is coronal view and rotated to visualize the needle
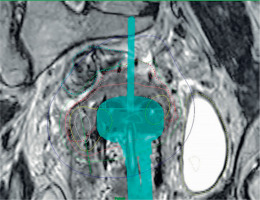
Using FHNs requires more skills and experience from the operator and dose planner. Because there is no guiding template or applicator holes, the needle insertion place, angle, and depth must be estimated from the images beforehand, and possibly by using intra-operative imaging, such as trans-rectal or trans-abdominal ultrasound imaging. FHNs can be more difficult to reconstruct. There is no help from applicator library images to identify needles. Additionally, FHNs technique might require pre-planning to determine the required number of needles and the optimal positions.
In the current study, two different type of applicators, i.e., Vienna™ and interstitial ring™ were used. In order to exclude any differences to dose coverage caused by the applicator, the impact of the applicator selection against FHNs plans and dosimetric data was verified.
In this work, the dosimetric differences between FHNs and APN techniques were investigated, especially the effect on target dose coverage and organs at risk (OARs) doses.
Material and methods
For this study, twenty-eight cervical cancer patients with FHNs at any fraction in their brachytherapy plans were considered. Fourteen patients had FHNs used in all fractions. Patients were treated between 2016 and 2020, and the average age was 65 (range, 36-88) years. From these patients, four cases were excluded. For such patients, plans would not have been possible to treat without FHNs. For example, tumor extension was inferior to the applicator ring, with no dwell positions in applicator, and these patients would have received EBRT boost without available FHNs option. Patient and plan data are presented in Table 1.
Table 1
Patient and plan characteristics
All patients received external beam radiotherapy (EBRT), 25-28 fx. × 1.8-2.1 Gy, and concurrent chemotherapy according to GEC-ESTRO recommendations [8, 9]. EBRT was either given in another national radiotherapy center (n = 19), or in a local center (n = 5).
After EBRT, patients were scheduled for 4 × 7 Gy brachytherapy boost. Applicator selection and needle configuration decision was made based on gynecologic clinical examinations and MR images from the time of diagnosis and at the end of EBRT. Applicator and interstitial needles were implanted under spinal anesthesia with spinal opioids as pain suppressor. Brachytherapy applicators used were interstitial ring™ applicator and Venezia™ applicator from Elekta. All needles were round-tipped plastic needles from the same vendor. FHNs were inserted through vagina or vulva before or after the applicator insertion, depending on target location and volume. No external template was used.
After implantation, treatment planning images were scanned using 1.5 T MR imager, with T2- and T1-weighted 3D image sequences and 1 mm slice thickness. Diffusion-weighted image sequence (DWI) was taken at the first fraction to assists in gross tumor volume (GTV) delineation. Tumor delineation was made by the same gyn-oncologist who performed the implantation. Organs at risk delineation and brachytherapy plans were made by a medical physicist. Tumor and OARs delineation and treatment planning was carried out with Oncentra Brachy™ software (Elekta AB, Stockholm, Sweden). Applicator and needle insertion, dose prescription, and fractionation were done according to GEC-ESTRO recommendations [10]. New applicator and needle implantation, imaging, and brachytherapy plan were made for each of the four treatment fraction. If needed, applicator configuration, number, position, or depth of the needles were adjusted in each following fraction. If the doses for targets and OARs were acceptable, no modifications were made at the next fraction.
All four plans for the 24 patients were retrospectively re-planned without using any FHNs. To simulate a real clinical situation, additional applicator needles were included, and existing needle depths were changed if the planner found that it was necessary to improve the dose coverage. The applicator type was not allowed to be changed. Oblique needles were employed if the applicator allowed it (Venezia™). Dosimetric constraints and targets were according to the department’s clinical practice and EMBRACE-II protocol [9] (Table 2). Dosimetric values from the treatment plans were converted to 2 Gy equivalent (2 Gy Eq) doses, by using α/β value of 10 Gy to targets, and 3 Gy to OARs. Doses from the four plans were then summed with EBRT dose distribution, and compared with doses from FHNs treatment plans.
Results
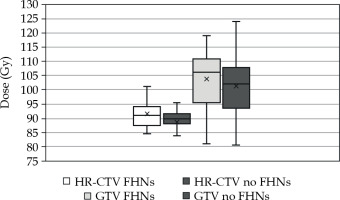
For HR-CTV, the mean 2 Gy Eq dose (α/β = 10) to 90% of the volume (D90) was 91.5 and 88.8 Gy with and without FHNs, respectively (p = 0.043). For GTV, the respective D98 values were 103.9 Gy and 101.3 Gy (p = 0.161). The average cumulative 2 Gy Eq doses for HR-CTV and GTV are presented in Figure 2.
Fig. 2
Cumulative 2 Gy Eq doses to the GTV D98 and HR-CTV D90 with and without free-hand needles (FHNs)
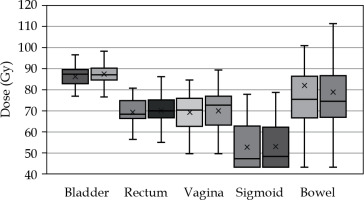
For OARs, the 2 Gy Eq mean doses (α/β = 3) to 2 cm3 volume of the whole delineated organ (D2cc) were: bladder = 86.2 Gy/87.5 Gy (p = 0.017), rectum = 69.2 Gy/70.2 Gy (p = 0.022), sigmoid = 69.3 Gy/70.1 Gy (p = 0.065), bowel = 52.6 Gy/53.1 Gy (p = 0.127), and vaginal wall point (recto-vaginal point) = 81.9 Gy/79.0 Gy (p = 0.137) with and without FHNs, respectively (Figure 3). The groups were compared with statistical one-tailed paired t-test, with statistical significance threshold being 0.05. In addition, we investigated how many plans without FHNs were violating hard dose constraints for targets and OARs defined in the EMBRACE-II protocol [11] (GTVD98 > 95 Gy, HR-CTVD90 > 85 Gy, bladderD2cc < 90 Gy, rectumD2cc, sigmoidD2cc, and vaginal point < 75 Gy). One plan had HR-CTVD90 < 85 Gy, with FHNs violating the hard constraint. After re-planning, in five treatment plans without FHNs, HR-CTV dose was < 85 Gy. With GTVD98, two plans violated the hard constraints originally and after re-planning without FHNs, the number increased to three.
Fig. 3
Cumulative 2 Gy Eq doses to organs at risk (OARs) with (left bar) and without (right bar) free-hand needles (FHNs)
When investigating in detail the planning aims, non-FHNs plans were inferior in almost every evaluated parameter. Planning aims were met with HR-CTVD90 dose in 11 of the 24 original plans and only 8 in non-FHNs plans. For the other parameters, the respective numbers were: GTVD98 = 18 and 16, bladderD2cc = 4 and 3, rectumD2cc = 5 and 5 (no change), sigmoidD2cc = 10 and 11, and vaginal point = 4 and 3. One plan was not treatable without FHNs, since D90 of HR-CTV coverage dropped from 96.8 Gy to 63.5 Gy, and GTV D98 decreased from 113.5 Gy to 58.6 Gy. The results are presented in detail in Table 3.
Table 3
Mean doses and hard constraint violations of targets and OARs with and without free-hand needles
* Constrains and planning aims according to EMBRACE-II protocol [1].
In this work, we also analyzed the impact of the applicator choice, i.e., interstitial ring™ and Venezia™, on dosimetric parameters with and without FHNs, number of FHNs used, and volumes of the targets. We did not find any statistically significant differences between the applicators and any of the analyzed parameters. The results of the impact of applicator selection are presented in Table 4.
Table 4
Applicator choice and impact on dosimetric parameters with and without FHNs
Acute and late complications were investigated retrospectively from the patients’ records. Two (8%) possible acute complications were registered, both suspected of needle perforation of the bowel, identified on MR images by a radiologist. However, none was clinically proven, nor required any treatment.
Late complication information was available for 14 patients (58%). Nine late complications were registered (64%), 5 of which were classified as grade 3+ (4 vaginal and 1 rectum complications). From all complications, vaginal problems, such as stenosis and stricture (5), and fistulas from vagina to adjacent organs (3) were most common. Also, two hydronephrosis were recorded. For the outcome analysis, 8 patients were lost to follow-up or died. From the remaining 16 patients, 2 had local relapse, one had stable disease, and one had progressive disease. Twelve patients achieved complete response, and are currently disease-free.
Discussion
Using FHNs with APNs is valid method of enhancing brachytherapy dose distributions in the treatment of cervix carcinoma. This is true especially in cases with extensive tumor burden after EBRT, when APN holes are not in the optimal places or angles. The use of few optimally placed FHNs may facilitate treatment plan, when other option is EBRT boost, which is not recommended option [11]. From outside of the analyzed patient cohort, we also managed to treat four patients with brachytherapy using FHNs. These patients would otherwise require EBRT boost or the use of a special applicator. On the other hand, FHNs technique may need more skills and experience from the physician, possible pre-planning, and intra-operative imaging, such as trans-rectal US or CT [12]. Without a template to guide the needle to a certain direction, it may be possible for FHNs to miss the target, or puncture bowel or another organ in its vicinity. However, this risk can be reduced by using a blunt tip needle, pre-defined insertion depth, and live image guidance, such as US or palpation, to position FHNs. Blind implantation of FHNs is not advisable. In addition, FHNs are usually more difficult to reconstruct in the planning phase than applicator guided needles, because of the non-defined insertion points.
In this work, we analyzed the advantages of FHNs in a situation, where only applicator needles were used. To our knowledge, there are only few publications on the use of FHNs in modern cervix cancer brachytherapy. In this study, we did not use any guiding templates or 3D-printed applicators while inserting FHNs. With few cases, trans-rectal US imaging was used to help positioning deep needles.
In a study by Qu et al., dosimetric advantages of distal parametrial free needles in cervix cancer brachytherapy were investigated [13]. The authors concluded that the treatment plans with free needles yielded higher doses to targets and smaller doses to OARs. However, they focused on the parametrial invasion, and the needles were inserted more distally through the skin of the perineum. Moreover, a Fletcher-type Utrecht™ applicator was employed, which has different APNs than a ring-type applicator used in our work.
Serban et al. explored the use of FHNs via 3D-printed template with excellent target coverage and OARs sparing [14]. This suggests promising results for truly individualized treatment. However, 3D printing for medical purposes requires human resources and technical expertise. Furthermore, printed material must be biocompatible and sterilizable, which means increased cost of 3D printer and more financial resources. Wang et al. used FHNs under CT guidance for large and bulky tumors without ring/ovoid part of the applicator in situ [12]. They used transperineal implantation when necessary, and found the technique feasible; although, DVH parameters were not inferior to the template-based studies. In addition, defining the target structure from CT images, especially GTV, is difficult.
During writing of this manuscript, a study by Jamadagni et al. [15] was published comparing free-hand technique and intra-cavitary technique dosimetrically. The authors reached the same conclusion that employing free-hand technique, the plans had statistically significantly higher D90 value of HR-CTV than using standard intra-cavitary technique. However, the intra-cavitary technique plans did not utilize any applicator-based interstitial needles; therefore, the plans were re-planned using only the applicator. This is obviously a limiting factor when comparing with IS needles plans.
Our patient cohort of 24 patients may be considered small, but represents the most challenging cases among all cervix BT patients treated in our department. For these cases, the standard applicator positions were insufficient and FHNs were required to achieve adequate dose coverage for complex target volumes. Consequently, it was not feasible to compare them against other patients with smaller and not so bulky tumors. The mean volume of HR-CTV within the selected patient group was very high (69.7 cm3) when compared with the EMBRACE-I group (HR-CTV mean volume of 28 cm3) [16]. From the results, we observed that using FHNs made a significant (p = 0.043) improvement of HR-CTVD90 dose. However, there was no difference in GTVD98 dose coverage. This is explained by GTV being often at the high-dose area, and FHNs are mainly helping to reach the far edges of HR-CTV. When investigating the significance of FHNs, five of the 24 studied patients would have not been treated without violating the most important hard constraint of HR-CTVD90 > 85 Gy. This could be a major clinical issue, and perhaps lead to change of treatment plan from BT to EBRT boost.
With OARs, statistically significant differences were found with the bladder and rectum (p = 0.017 and 0.022, respectively). The use of FHNs resulted in less dose to all delineated organs, except for the vaginal point, although that was not significant (p = 0.137). In addition, the vaginal point doses were highly influenced by the tumor burden at the bottom of the vagina. With all the patients, 50% were violating the vaginal wall (recto-vaginal point) hard constraint of < 75 Gy. The treatment plans were approved by the physician based on the tumor location and individual clinical situation.
Lastly, we investigated if the applicator type had an impact on FHNs usage or doses. About half of the patients were treated with interstitial ring™ applicator, which has no holes for oblique needles, but has a central opening. The other half of the patients were treated with Venezia™ applicator, with the possibility of oblique needles. From the results, it can be seen that the applicator selection had no impact on the number of FHNs or dosimetric values in original plans or re-planned plans. In practice, our clinic uses the open ring of interstitial ring™ applicator to reach deeper into the uterus than the IU tandem permits. This is not an option in Venezia™ applicator, because of the closed ring. Instead, the Venezia™ applicator has longer IU tandem available than the interstitial ring™. Both applicators have a limit of 5 cm insertion depth with APNs. This might partly explain the lack of difference between the applicators.
In the current study, the number of acute complications was very low. No excess internal or external hemorrhage due the needles was observed. Two radiologically susceptible bowel perforations were noted on the planning MRIs: the first was inserted from the lateral side of ring and the other through the central opening. These cases were finally concluded that the needle tips were pressing hard against bowel surface, but not actually perforating it. To avoid perforations, all needles were round-tipped. Symptoms, such as infections etc. from possible perforations were not recorded.
In addition, late complications were analyzed. Because only 21% of the patients were local patients, it was challenging to obtain follow-up data retrospectively. From the information gathered, we noticed that late complications were common, which was expected in this patient group of large and difficult tumors. Most common late effects were complications of the vagina (4), fistulas (3), and hydronephrosis (2). From severe complications (grade 3+), fistulas were most common. Because the observed fistulas were at the locations where the tumor was already invading the boundaries of the organs, we deduced that these late effects were most probably not due to the use of FHNs.
From the analyzed 24 patients, half of them (12) are presently disease-free; two patients had local relapse and 8 were lost to follow-up or died. Since this group included mostly patients who were originally planned to avoid brachytherapy boost for being too difficult to perform, the results of the study can be considered good.
Conclusions
In this study, we compared real brachytherapy plans with FHNs and hypothetical re-planned versions without FHNs in the group of patients with large cervical carcinomas. Although the number of patients was small, we proved that combining FHNs with the commercially available advanced cervix brachytherapy applicator, increases D90 dose to HR-CTV and decreases doses to critical organs. Tailored positioning of FHNs also showed to be feasible without expensive custom 3D-printed applicators or live image guidance. FHNs technique requires experience and skills from the physician and physicist, but it may enable the use of brachytherapy instead of EBRT in very difficult patients.



