Purpose
Low-dose-rate brachytherapy (BT) with permanent iodine-125 (125I) radioactive seeds is a highly effective treatment option in patients with low-risk and favorable intermediate-risk prostate cancer. It equally allows for both long-term local and biochemical control compared to those observed after radical prostatectomy or external beam radiation therapy [1]. With high survival rates associated with these techniques, patients usually make a treatment decision based on their understanding of toxicity risk differences between each treatment modality.
In prostate BT, optimal planning after implantation is not always achieved due to prostate volume changes or seeds loss [2]. In addition, periprostatic and extra-glandular seeds placement may cause source migration in a small percentage of patients [3,4]. One way to improve seed placement is the use of stranded seeds called “intraoperatively built custom-linked seeds (IBCLS)” in an opposition to loose seeds (LS). The IBCLS system allows the user to create customized linked seeds, with seeds connectors and spacers [5]. Previous studies comparing both procedures have shown a more homogeneous dose to the prostatic gland with IBCLS, with a lower dose to organs at risk than those obtained with LS. However, very few data comparing acute and late toxicities between these two techniques are available in the literature.
The aim of this study was to compare the acute and one-year toxicities of both procedures in a matched-paired population.
Material and methods
Patients
This work is a mono-centric retrospective study with prospective assessment. Between 2003 and 2018, 548 patients were treated with permanent prostate low-dose BT with 125I seeds for a low-risk or a favorable intermediate-risk adenocarcinoma prostate cancer, according to D’Amico classification at our institution. Patients were considered for BT with the following criteria: 1. Gleason score < 7 with prostate-specific antigen (PSA) < 10 ng/ml and < T2b, or PSA 10-15 ng/ml or T2b, or Gleason score 7 (3 + 4); 2. ≤ 50% of positive biopsies; 3. Initial prostate volume ≤ 60 cc as measured by transrectal ultrasound [6]. Patients with previous external radiotherapy to the pelvis or those who received androgen deprivation to decrease prostate volume before the procedure were not considered. Patients were included in the analysis if they have completed at least 24 months of follow-up.
A matching process using the Excel® match function was undertaken from the entire patient cohort to produce two similar groups of patients, depending on the brachytherapy seeds technique. Patients in the LS cohort were individually matched to patients in the IBCLS cohort. We did not consider the first 40 patients in the loose seeds group in order to exceed the learning curve [7]. On the opposite, the IBCLS procedure does not require a learning curve after an LS experience [8]. Matching was performed according to the following factors: age, prostate volume, pre-operative international prostate symptom score (IPSS), clinical stage, and Gleason score. Weights were assigned in order of importance on the IPSS, TNM stage, prostate volume, Gleason score, and age. Because the number of patients treated with IBCLS (n = 123; 13 patients without at least 24 months of follow-up regarding the date of implantation seeds) was smaller than that of patients treated with LS (n = 425), 110 patients were randomly selected from the LS group to match the distribution. When a matched-pair could not be obtained, patients were excluded from the analysis. After several repeats of matching and exclusion, 105 patients with brachytherapy procedure using LS and 105 matched patients with IBCLS were selected for the study population.
Treatment
The choice of treatment was made by the patient after clear and fair information of the benefits and side effects of each available curative option. All patients underwent an ultrasonography volume study at 2-6 weeks before the implantation to determine the number of seeds.
Iodine-125 BT procedures were performed by the same experienced team, including an urologist, a radiation oncologist, and a physicist, using real-time dynamic dosimetric planning, as described by Stone and Stock [9], with patients in an extended lithotomy position and under general anesthesia. Using a transrectal ultrasound probe attached to the stepper, transverse images from the base to the apex of the prostate were captured at 5-mm intervals and were loaded into the treatment planning software (Variseed, Varian Medical Systems, Palo Alto, CA, USA). The prostate (excluding seminal vesicles), urethra (with a balloon catheter), and rectal wall were outlined. Interstitial needles were inserted into the prostate through a perineal template under sagittal and axial transrectal ultrasound image guidance. Each needle location was registered in real-time on the treatment planning computer. A secondary image capture was performed after needle insertion, and the contours of all of the structures were revised. Next, a dosimetric plan, which accounted for prostate movement and swelling after needle insertion, was generated.
Once the plan was approved, 125I loose seeds or intraoperatively custom-linked seed trains were manually placed into the prostate. In our institution, loose seed were used up to 2016, and were then replaced by IBCL, which combines seeds and connectors into seed trains of variable length, with variables seed-to-seed spacing as predetermined by planning.
Endorectal ultrasound imaging allowed a real-time visualization of the implantation to assure appropriate placement of the seed/train. The computer provided a real-time dynamic dosimetric evaluation of the implant, allowing the plan to be corrected during the procedure if needed.
The technique of 125I BT was carried out, with a prescribed dose of 160 Gy. The dose constraints used for organs at risk were as follows: for the urethra: D10 < 218 Gy, D30 < 188 Gy; for the rectum: D2cc < 145 Gy, D0.1cc < 200 Gy. To assess the reproducibility of dosimetric plan, the ΔD90, which is the absolute change in the minimum dose received by 90% of the prostate volume between the intraoperative and post-operative planning, was calculated (ΔD90 = post-operative D90 – intraoperative D90). If the absolute change in prostate D90 (ΔD90 = post-operative D90 – intraoperative D90) is low, the plan reproducibility is considered to be high [10].
Post-implantation dosimetry
On the operative day, patients underwent a cystogram as well as a chest X-ray to exclude bladder and lung seed migration. Patients were requested to strain their urines during the first 15 days after the procedure.
One month after the implantation, patients underwent a pelvic computer tomography with 2-mm slice images (Philips Aura CT Scanner, Philips Medical Systems, Best, The Netherlands) [11]. The images were uploaded into the Variseed v.9.0 treatment planning system (Varian Medical Systems, Inc., Palo Alto, CA, USA), and the prostate and rectum were then outlined. During the implantation, the urethra was defined based on the urethral catheter, and was contoured from the bladder neck to the prostatic apex under sagittal and axial transrectal ultrasound image guidance. The patients were not catheterized for the post-operative CT scan, therefore, only the peri-operative dose to the urethra was reported in this study. The location of the BT seeds was defined by a combination of manual and automated redundancy checks available on the Variseed software system.
Toxicity assessment and follow-up
Toxicities were assessed using the international prostate symptom score (IPSS) [12] and the five-item international index of erectile function (IIEF-5) [13]. IPSS class were considered as follows: mild (0-7), moderate (8-19), or severe (20-35). The IIEF-5, also known as SHIM (sexual health inventory for men), is a validated self-administrated questionnaire, which allows to classify erectile dysfunction in different classes according to the score obtained, including no erectile dysfunction (ED): > 21, weak ED: 21-17, weak to moderate ED: 16-12, moderate ED: 11-8, severe ED: < 8. The clinical impact of erectile toxicity was reported by the change in IIEF-5 class between the pre-operative IIEF-5 (if superior to 16) and IIEF-5 at first and second year. Rectal symptoms were scored using the common terminology criteria for adverse events (CTCAE) v.4 [14]. All patients started α-1 adrenergic antagonist therapy immediately after BT, for a 6 months duration. As patients after LDR brachytherapy fail to improve quality of life within 12 months after seed implantation [15], we do not routinely prescribe cyclooxygenase-2 (COX-2) inhibitors.
Statistical method
Descriptive statistics were used for patients’ demographics, procedures characteristics, dosimetric variables, CTCAE toxicities, and IPSS and IIEF-5 scores as well as clinical outcomes. The normality of the IPSS distribution was tested using Shapiro-Wilk test. The mean and standard deviations of questionnaire scores at baseline and follow-up were calculated. A multivariable linear mix-effects model was used to describe the change of IPSS, including prostate volume, age, brachytherapy technique, and time to visit, as covariates. Statistical significance was defined as a two-tailed p-value < 0.05. All analyses were conducted using a R stat software version 3.6.0.
Results
The median follow-up of the entire cohort was 83.3 months (range, 29.5-207.4 months). Patients characteristics of the entire group are displayed in Table 1.
Matched-paired patients and tumor characteristics
There were no differences in patients’ characteristics between the two groups after the matched-paired procedure (Table 2). For the entire cohort, the mean age was 64.87 years, and 61 (29%) patients presented with a favorable intermediate-risk prostate cancer. Seven patients were lost to follow-up and were censored at date of last available PSA.
Table 1
Patients characteristics of the whole cohort
Table 2
Patients’ characteristics
Dosimetric considerations
The IBCLS technique required fewer needles (21.4 vs. 18.4, p < 0.001), but a similar number of seeds (64.5 vs. 62.3, p = 0.068) than the LS procedure. Seeds migration to the lungs occurred in one patient in the LS group and one patient in the IBCLS group. No patients reported seeds loss after the procedure. There was no difference in peri-operative parameters D90Gy (180.0 Gy vs. 179.8 Gy, p = 0.65) and V100% (96.7% vs. 96.7%, p = 0.93), in the LS and IBCLS groups, respectively, but a lower V150% in the IBCLS group (51.9% vs. 48.8%, p < 0.001). At 1 month, D90Gy, V150%, and V100% were higher in the LS group compared to the IBCLS group (166.2 Gy vs. 150.4 Gy, p < 0.001; 60.5% vs. 52.1%, p < 0.001; 92.2% vs. 90.7%, p = 0.003, respectively) (Table 3).
Table 3
Dosimetric parameters in each investigated group
The reproducibility of the dosimetric plan was better in patients treated with loose seeds, with a mean ΔD90 of –13.6 ±16.43% in the LS group compared to –29.3 ±8.02% in the IBCLS group (p < 0.001).
Regarding organs at risk, the peri-operative doses to the rectum and urethra were higher in the LS group compared to the IBCLS group. The maximum dose to the rectum and urethral D10% were 113.5 Gy vs. 107.8 Gy, p = 0.002, and 190.5 Gy vs. 185.8 Gy, p < 0.001 in the LS and IBCLS groups, respectively (Table 3). At one month, there was no difference in D2cc to the rectum (108.4 Gy vs. 109.2 Gy, p = 0.539) or in D0.1cc (179.6 Gy vs. 185.5 Gy, p = 0.384).
Toxicity outcomes
Questionnaires of IPSS and IIEF-5 were completed by all patients before BT, at one, and 3 months after the procedure, and every 6 months thereafter for 5 years.
Patients in the IBCLS group had a lower IPSS than those in the LS group at three month (13.1 vs. 15.6, p = 0.020) and at one year (8 vs. 10, p = 0.022) (Figure 1, Table 4). Change for a superior IPSS class at one year occurred more often in patients treated with LS (43% vs. 27%, p = 0.027), but at 1, 6, 18, and 24 months post-procedure, IPSS scores were similar in the two groups, with no difference in the consumption of alpha blocker at 1 year (32% vs. 25%, p = 0.43 in the LS and IBCLS groups, respectively). According to the linear mixed-effects model, there was no significant IPSS changes over time (p = 0.57). Two patients in the LS group and one in the IBCLS group experienced acute urinary retention.
Fig. 1
Average value of IPSS (international prostate symptom score) in the IBCLS (intraoperatively built custom- linked seeds, blue bars) group and the LS (loose seeds, orange bars) group before brachytherapy and during follow- up. Errors bars display the range of values. Data are normally distributed (according to Shapiro-Wilk test)
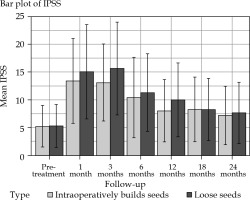
Table 4
Toxicity occurrence in each group
Erectile dysfunction was similar in the two groups, except at one month, where the IIEF-5 was lower in the IBCLS group (10.9 vs. 6.9, p = 0.029).
Digestive toxicity was also assessed at each follow-up visit. Grade ≥ 2 rectal toxicity was observed at 1 month (1%) in 2 patients in the LS group (Table 4) vs. none in the other group. A transitional difference was observed at 6 months in favor of the LS group; indeed, the rate of grade 1 toxicity was 16.1% in patients treated with IBCLS vs. 4.7% with LC (p = 0.008). However, from one year on, no difference was noted between the 2 groups.
Discussion
To our knowledge, this is the first study comparing toxicity outcome between stranded seeds and LS for low-dose-rate prostate BT at 24 months after the BT procedure.
Toxicity outcome after BT are highly correlated with the existence of pre-operative urinary symptoms. Here, the mean pre-implantation IPSSs observed in each group are consistent with the literature in both the LS (5.3) and the IBCLS (5.2) cohorts, and were comparable between the 2 groups as IPSS was a criteria for matching. Zuber et al. reported a mean IPSS of 6.3 ±4.0 in a population of 169 patients before treatment, but 25% of them received neoadjuvant androgen deprivation, which is known to improve urinary symptoms [16]. In another study on 195 patients, who did not receive hormonal treatment, the mean IPSS before treatment and at one year post-therapy was 4.5 ±3.8 and 7.9 ±5.6, respectively [17]. In our study, patients in the IBCLS group had a significantly lower IPSS at 12 months compared to patients in the LS group (8.03 vs. 10.00), with no difference in the consumption of alpha blockers at 1 year (32% vs. 25%, p = 0.43). This may be explained by the lower urethra dosimetric parameters, especially the D10% and D30% obtained with IBCLS compared to LS. However, an opposite results were reported by Major et al., who observed less intraoperative dose to rectum and urethra with LS compared to stranded seeds, but a slightly worse target coverage [18]. Recently, anatomic subregions have been identified as involved in ureterovesical symptoms, namely the posterior part of the bladder, which dose was shown to be predictive of late retention and dysuria in external radiotherapy [19]. As less local seeds migrations are observed with the IBCLS technique [8,11], it can be hypothesized that lower dosimetric parameters to the urethra and less irradiation of the bladder mucosa result from the use of IBCLS [3].
Grade 2 or more rectal toxicity is rare after LDR-BT, and usually ranges between 0 and 2% [20]. Ohashi et al. reported a 3-year cumulative incidence rate for grade ≥ 2 late rectal toxicity of 0.90% in a large cohort of 2,339 patients [21]. In this regard, the grade ≥ 2 toxicity rate of 1.9% at 2 years reported in the present study is in line with the literature. Saibishkumar et al. reported a higher dose to the rectal wall and a slight increase in acute rectal grade ≥ 2 toxicity in patients treated with IBCLS compared to patients with LS, but his study included only 40 patients [22]. We found G1 toxicity to be slightly higher at 6 months in the IBCLS group, although post-operative parameters were similar in the two groups. G1 toxicity rates at 3, 12, and 24 months were comparable between the two groups, therefore, we can assume that the difference observed at 6 months may not be clinically significant.
It is known that patients treated with 125I low-dose-rate BT have overall very few complications [23]. During the first 4 years after BT, more than a half of patients maintain an IIEF-5 > 16, with very rare severe erectile dysfunction [24]. In a series of 2,928 patients, erectile function was even preserved in more than 80% of young patients at 5 years [25]. Several parameters can influence potency after BT, and the dose to the penis bulb might be of importance [26]; after a 2-year follow-up, among patients who had no or weak erectile dysfunction (IIEF-5 ≥ 16) at baseline, 62% in the LS group and 59% in the IBCLS group presented no clinically relevant decrease in erectile function. The follow-up was however too short to assess the long-term effect on erectile function, as BT-related erectile disorders may occur after 2 years after implantation [25].
In our study, the reproducibility of the dosimetric plan was better in the LS group, illustrated by the ΔD90 (–13.6 ±16.4% and –29.3 ±8.0%, p < 0.001), which is explained by a lower D90 at 1 month in the IBCLS group. There was no clinical impact of this difference, but the follow-up was too short (especially in the IBCLS group) and the number of relapses too small to draw any conclusion. In a recent dosimetric study performed by Kaneda et al., there was a trend towards a lower post-operative D90, V150, and V100 with IBCLS compared to LS (118.8% vs. 127.2%; 51.7% vs. 66.7%; 0.44 ml vs. 0.61 ml, respectively, p < 0.01) [10]. Another retrospective study showed that procedures with LS were associated with intraoperative lower doses to the urethra and rectum compared to IBCLS [18]. In the opposite, Ishiyama et al. did not observe any post-operative dosimetric difference between IBCLS and LS in a prospective trial [27]. In our study, IBCLS allowed significantly lower dosimetric parameters to organs at risk; although, this difference did not appear on the post-operative planning.
IBCLS technique has advantages compared to LS. Activity required to treat a prostate of a given volume is lower with IBCLS than with LS [28]. Moreover, less post-implant seed migration have been observed when using the IBCLS technique [27,28,29], especially in the chest BT: HR = 7.9 (2.3-28.1) vs. HR = 15.9 (5.9-42.1), p < 0.0001, per 1,000 permanent prostates [11]. Using a coated vicryl to the surface of radioactive seeds may help to lower propensity of the seeds to slip from their initial implant location, thus maintaining dosimetric integrity and reducing lung and pelvic seed migration [30]. Moreover, coated seeds have a significant anchoring effect, which is particularly effective in reducing the number of apical seeds loss [2].
A multi-institutional retrospective analysis showed no dosimetric differences and no learning curve, but an extended operation time by up to 7 min for IBCLS [31]. We did not consider the first 40 patients treated with LS in order to exceed the learning curve, as a significant difference in the post-implant D90 exists for patients who received their implants after 40 procedures [6]. A very short learning curve of about five patients has been reported for IBCLS after an LS experience [31].
Our study has the advantage of having comparable homogenous populations obtained through the matching procedure. However, it is mono-centric and retrospective in nature, with a limited number of patients and short follow-up. Indeed, it is known that IPSS significantly increases at 3 months following BT and returns to baseline by 36 months. However, in the recent study by Onishi et al., although total IPSS decreased between 12 and 36 months (10.1 to 8.81), the scores at 2 (9.89) and 3 years (8.81) were not significantly different from baseline (7.99) [32]. Similarly, Ohashi et al. found that the mean of post-implant IPSS peaked at 3 months, but decreased to a range that was within 2 points of the baseline score at one year in 70% of patients, in a cohort of 1,625 men treated with LDR-BT [21]. Thus, this allows an assumption that IBCLS would not be associated with higher urinary toxicity at a later time point. We also acknowledged that urinary toxicity was reported using the IPSS only, although patients can present with other symptoms, such as painful burning sensation during urination. The storage symptom, for instance, does not return to baseline at 36 months post-BT [32].
During this short follow-up, 3 patients presented a biochemical recurrence, with one in the LS group and two in the IBCLS group, all of them at 2 years post-implantation. This has already been studied by others. Herbert et al. did not find any difference in biochemical control between LS and IBCLS in 1,500 patients [33]. In another study by Hinnen et al. on 896 patients, those treated with LS presented less biochemical control, but androgen deprivation was allowed and could have biased results [34].



